| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
December 4, 2010 – Stem cells are known mainly for their ability to self-renew and to give rise to cells of different types, but many have a third important characteristic as well: they can shut down, becoming “quiescent.” This is particularly crucial for stem cells that function in the adult body, as they need to be able to maintain tissue homeostasis by dividing to produce progeny cells in precisely the right numbers to maintain integrity without over-proliferating. This dormant state has been studied mainly in term of cell cycle regulation, cell metabolism, and interactions with the stem cell niche. More recent evidence suggests that transcriptional activity is also suppressed in quiescent stem cells, but the mechanisms by which this slowdown is achieved have yet to be identified.
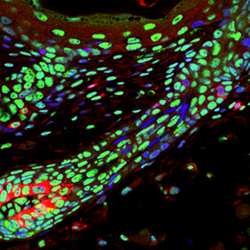
Now, a study by Rasmus Freter and others in the Laboratory for Stem Cell Biology (Shin-Ichi Nishikawa, Group Director) has revealed the mechanistic basis for stem cells’ transcriptional slumber. Published in the journal Stem Cells, this new work reveals that adult stem cells globally suppress the phosphorylation of a specific amino acid in the tail-end of the crucial enzyme, RNA polymerase II (RNApII), in mouse. Freter, now at the University of Oxford, began the study knowing that mRNA transcription is markedly reduced in quiescent melanocyte stem cells. Looking for the underlying mechanism, he tested for the phosphorylation of serine 2 in the C-terminal domain of RNApII, which is known to trigger elongation, a necessary step in the synthesis of mRNA. While melanocytes and migrating progenitors stained positive for phosphorylated ser2, this was downregulated in stem cells that had settled into their niche in the hair follicle. Quiescence is typically induced when a stem cell is deprived of survival factors, which in the case of melanocyte stem cells include SCF/c-Kit. To determine whether a relationship existed between this stem cell factor and phosphorylation of this amino acid, the group used a transgenic mouse in which SCF is constitutively overexpressed. They found that the melanocyte stem cells in adult animals lacked phosphorylated ser2, but interestingly, that this was present at the time of birth. Apparently a short-lived signal caused dephosphorylation in the immediate postnatal period, but was later overridden by the constitutive effect of SCF in the mutant animals. Looking for clues behind the dephosphorylation in the transgenic adults, Freter analyzed the expression of the cyclin-dependent kinase p-TEFb, which is composed of CDK9 and a cyclin partner, and is known to trigger the phosphorylation of ser2. He found that while CDK9 was unperturbed in differentiated cells, it was specifically downregulated in melanocyte stem cells, presumably as a result of the global transcriptional suppression that takes place in these cells. This supposition was lent credence by the finding that conditional knock-in of CDK9 did not induce phosphorylation in these melanoblasts. The group further found that the inhibition of CDK9, used here as a readout of the phosphorylation status of ser2, increased the survival of cultured cells during growth factor starvation by suppression of cell death pathways. To determine whether the role of ser2 phosphorylation in the C-terminal domain was common to quiescent stem cells in other tissues, the group examined a range of other stem cell types, including satellite cells in muscle, spermatogonia stem cells and blood-producing hematopoietic stem cells, in addition to the melanocyte and keratinocyte stem cells from the hair system. They found that in all cases, the inactive stem cells showed lower ser2 phosphorylation than that seen in actively cycling cells, suggeting that global transcriptional suppression may be a general feature of stem cell quiescence. “Downregulation of CTD Ser2 phosphorylation is beneficial for cell survival when cells are confronted by stress, and thus maintains the stem cell compartment throughout the life of the organism,” says Freter. “The detection of transcriptional quiescent cells in organs, or even cancer tissues, may therefore be useful in the identification of cells with stem cell-like properties." |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |