| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
July 4, 2011 –The developing mammalian brain is characterized by rapid proliferation and differentiation, needed to generate the diversity of neural cells and tissues that make up the adult organ. During this stage, one cellular population known as radial glia cells plays the part of neural stem cells capable of both self-renewal and the generation of differentiated progeny. These cells feature processes at both their apical and basal ends, which are allocated asymmetrically to daughter cells in a context-dependent manner, but the relationship between such segregation and the ability to self-renew remains poorly understood.
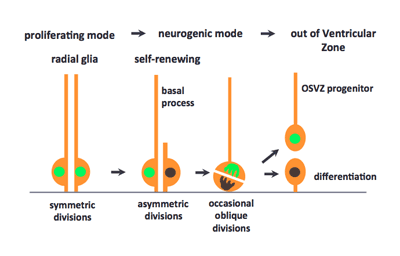
New research by Atsunori Shitamukai and others in the Laboratory for Cell Asymmetry (Fumio Matsuzaki, Group Director) now sheds new light on the angle of cell division as a crucial determinant of daughter cell fate, with progeny produced by oblique divisions assuming a new combination of differential fates depending on whether they inherit the apical or basal process. Published in The Journal of Neuroscience, these findings are in strong agreement with previous work in the primate nervous system, suggesting that the mouse can serve as a useful model for more detailed studies of this neurodevelopmental process. The group began by observing the fates of daughter cells from apical and basal progenitors, using an electroporation system previously developed by the lab, which enabled them to distinguish between daughter cell types they called apical and other progenitors, after the location at which their subsequent cell division occurs. Apical progenitors, or radial glia cells, divide at the apical surface after interkinetic nuclear migration (INM) and form the apical junction, while basal progenitors divide a single time in the subventricular zone (SVZ), giving rise to a pair of neurons. The group tested whether the fates of daughter cells in such divisions was dependent on the size of the inherited apical membrane, as had been suggested by some previous studies, but found that in this context the question of self-renewal vs. differentiation was unaffected by differences in inherited domain size. Shitamukai next examined whether the inheritance of the basal process played a role. All apical progenitors divided asymmetrically with regard to this structure, meaning only a single daughter inherits it. By monitoring subsequent changes in the morphology and behavior of daughter cells that had or had not inherited basal process, they were able to determine that those cells that inherited a process often maintained their ability to self-renew, becoming either apical or nonsurface progenitors; daughter cells that did not inherit the basal process showed no such proliferation. Expression of genetic indicators of Notch activation, a hallmark of self-renewal ability in these cells, again showed that inheritance of the basal process is associated with the generation of self-renewing progenitor cells. Shitamukai et al. next employed a strategy previously developed by the Matsuzaki lab for genetically perturbing the orientation of the cleavage plane, to examine the respective roles of the apical and basal domains on daughter cell fate. They found that such misorientation at apical divisions induces progenitors outside the ventricular zone (outer VZ progenitors) that retain the basal process and, unlike basal progenitors, are capable of self-renewal. Analysis of marker genes for differentiation and self-renewal further supported these findings.
Previous work by the lab had shown that even in normal mouse brain slices, there are occasional progenitor cell divisions in which only a single daughter inherits the apical domain. To determine whether these basal-only daughters in wild type slices become outer VZ progenitors as well, the group observed unmodified both live brain slices and fixed brain sections and found that, in this context as well, there is a small population of outer VZ progenitors that retain the basal process and proliferative capacity. Interestingly, the survival of these progenitor cells depends on Notch signaling with their own progeny cells. Neural progenitor cells that are equivalent to outer VZ progenitors, known as either outer subventricular zone (OSVZ) progenitor or outer glial cells, have been identified in the developing brain of gyrencephalic mammals, including ferrets and primates. In primates in particular, these OSVZ progenitors predominate among self-renewing neural progenitor cells from mid-neurogenic stages onwards, and generate most of the neurons in the upper layers of the neocortex. Therefore, the appearance of OSVZ progenitor cells has been thought to be important in the evolution of the brain, as a cause of the expansion in brain size and the consequent emergence of brain gyration in these species. Some have speculated that rodents, which are agyrencephalic, might lack OSVZ progenitors as well. Shitamukai et al., however, have shown that the OSVZ type of progenitor cells had already evolved in the rodent taxa, although their population is smaller than that of radial glial cells. The group also proposes how OSVZ progenitor cells are generated in primates, suggesting that they may be born from radial gilal cells by oblique apical divisions in which one daughter cell loses the apical domain, as happens in mice. Matsuzaki notes, “A number of human hereditary diseases are thought to be caused by abnormal behaviors of neural progenitors. Some of these may be due to abnormalities in OSVZ progenitor cells, which would have made them difficult to study experimentally, as this is a minor population in mice. Our LGN mutant mice generate a number of the OSVZ progenitors comparable to that of radial glial cells, which makes it possible to analyze some properties and genetic defects of the OSVZ progenitors by using this mouse strain as a model.” | |||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |