| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
July 22, 2011 –The uneven distribution of cellular components is a fundamental process in development and differentiation, as can be seen in the sequestration of maternal RNAs and proteins in a form of cytoplasm known as germ plasm at the posterior pole of the Drosophila oocyte, which is allocated only to daughter cells that go on to form the germ lineage. Failure to maintain this germ plasm at the egg’s polar region results in defects in germ cell formation, highlighting its critical importance to the fly’s reproductive viability. The means by which the germ plasm is anchored to the oocyte pole, however, remain incompletely understood.
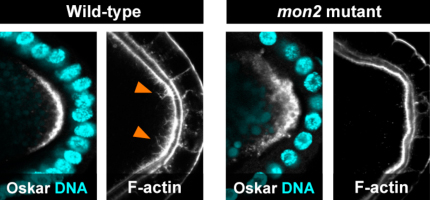
Tsubasa Tanaka and colleagues in the Laboratory for Germline Development (Akira Nakamura, Team Leader) have now worked out at least part of this mystery, finding that the protein Mon2 works with a second factor, Oskar (Osk), to remodel actin and hold the germ plasm in its proper place. Published in Development, this work may shed light on more general principles of the generation of cellular polarity. The role of Osk in germ plasm assembly is well established – osk RNA is carried to the oocyte’s posterior pole where it is translated, forms complexes with other polar components, and triggers the remodeling of F-actin at the cell cortex. The resulting actin fibers serve as anchor points in the polar region. The Nakamura team previously found that Osk appears to accomplish this feat through the activation of the endocytic pathway, a mechanism by which cells absorb and recycle molecules on the cell membrane, but the mechanistic link between this process and actin remodeling remained uncertain. To investigate more closely, Tanaka began by identifying six mutants in which polar factors such as Osk and Vasa failed to stay localized at the poles. Analysis of the underlying genetic changes revealed that all six were caused by mutations in the gene encoding Mon2. When they looked at the patterns of Mon2 expression in the oocyte, they found that while it began as a punctate expression throughout the egg, it gradually shifted to the cortex, resembling the localization of Golgi and some endosomes. The team next asked what function Mon2 might have in maintaining the germ plasm at the pole. In the mutants, they found no changes in microtubule assembly, needed to convey pole factors to their destinations, nor in the ability of Osk to activate endocytosis. But when they looked at its effect on actin, they found that Mon2 loss of function interfered with the cytoskeletal component’s remodeling. Analysis of genetic interactions between Mon2, Osk and the endocytic regulator Rab5 revealed that Mon2 operates downstream of both Osk and the endocytosis it triggers. When Tanaka tested for protein binding partners for Mon2, he found that it physically associated with a pair of actin nucleators, Cappuccino (Capu) and Spire (Spir). Interestingly, Spir includes a Rab-binding domain, pointing to a possible interaction with endosomes. Loss of Capu and Spir function resulted in the loss of actin projections at the polar cortex; the actin fibers instead accumulated in fuzzy ball-like accretions, indicating a failure of the actin remodeling requisite for germ plasm tethering. Knowing that both Spir and Capu interact with Rho1, a protein known for its roles in actin regulation, the team next tried to copy the loss of Capu and Spir phenotype by interfering with Rho expression, which resulted in the expected failure of actin remodeling. They further found that in Mon2 mutants, Rho’s normal localization at the oocyte posterior pole was lost. The picture that develops is one in which the activation of endocytosis by Osk spurs actin remodeling by Mon2 downstream of Spir, Capu and Rho, thereby providing an anchorage for the germ plasm. “There have been a number of recent indications that the endosome serves as a platform for the assembly of molecular complexes, as well as other lines of evidence that show that intracellular vesicles, such as endosomes, play a role in ferrying molecules through the cell,” says Nakamura. “Given the importance of these functions, what we have learned about Mon2 in this study may have implications for the generation of polarity in other contexts as well.” | |||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |