| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
September 15, 2011 –Cells have a well-developed sense of their surroundings, identifying and interacting with many diverse components of their environments. This is clearly seen in the proliferation of cells in culture – a population will grow steady until the cell density crosses a given threshold, triggering a shutdown. This phenomenon, known as “cell contact inhibition of proliferation,” has been tied to the Hippo signaling pathway, but the molecular mechanism remains mysterious. In specific, it is unknown whether the key cue behind this process is cell morphology or cell-cell adhesion, or some combination of the two.
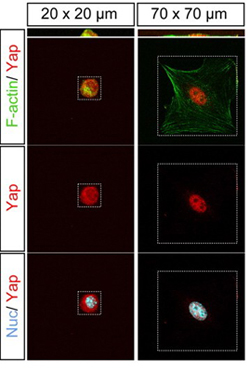
Now, a study by Ken-ichi Wada and others in the Laboratory for Embryonic Induction (Hiroshi Sasaki, Team Leader) looked at individual cells to examine the effects of cell morphology on Hippo signaling in isolation from other influences. Published in Development, the report describes how cell shape and underlying cytoskeletal proteins, such as F-actin, regulate the Hippo pathway in vitro. Both Wada and Sasaki have since moved to the Kumamoto University Institute of Molecular Embryology and Genetics. In a previous study, the Sasaki team had shown how changes in cell density control Hippo activity; Hippo is down-regulated at lower densities, resulting in the nuclear accumulation of a coactivator protein, Yap, increasing activity of Tead, a transcription factor that promotes proliferation. Conversely, at higher densities, Hippo is activated causing the phosphorylation and cytoplasmic retention of Yap, preventing Tead activity and, as a result, stoppage of proliferation. This report, however, left open the question of how Hippo signaling is able to detect the density of cells in an environment. Numerous studies have shown links between cell-cell adhesion and regulation of the Hippo pathway, and other reports have described the effect of cell morphology on proliferation, which led Wada to speculate that cell shape might also play be a missing piece in the Hippo–proliferation puzzle. In order to study this, however, he first needed an experimental system that would allow him to examine cells in isolation from contact with other cells. By culturing cells in microdomains of different sizes, he was able to grow single cells in spatial constraints that affected their morphology. In smaller 20 × 20 domains, the cells assumed a compact, rounded form, while in roomier 70 × 70 quarters, they became flattened and spread out. Looking next at the expression of the Hippo pathway factor Yap, he noted that in cells grown in the smaller domains, Yap remained outside the nucleus, while in those cultured in the larger domains it was localized in the nucleus, suggesting that morphology can act as a regulator of the distribution of this protein.
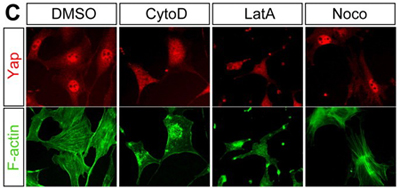
But how is this regulatory control achieved? The key may lie in a form of F-actin known as stress fibers. In cells cultured in the 70 × 70 domains, as a model of lower cell density, stress fibers were abundant and present throughout the cells, while in the 20 × 20 domains their distribution was punctate and only faintly detectable. To determine whether the apparent correlation between stress fiber quantity and cell density works in Hippo regulation, the team next disrupted stress fiber formation using an actin-inhibiting agent. As expected, this caused Yap to localize in the cytoplasm as occurs in high-cell-density environments. This latest work form the Sasaki team cements a role for cell morphology in the regulation of proliferation mediated by the Hippo pathway. “It will be interesting to tease out how Hippo signaling integrates diverse information inputs relating to a cell’s shape and its adhesive contacts with neighboring cells,” says Sasaki. “We know that actin is also involved in cell-cell adhesion, so that may be one key to understanding the link to Hippo. We are also very interested in the role that Hippo plays in controlling differentiation in the preimplantation mammalian embryo, and whether these latest findings will prove to be important to our understanding of early development as well.” | |||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |