| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
March 20, 2012 –Brains, whether they belong to flies, mice, or humans, are built of numerous types of neurons and supporting cells. This great diversity is the result of asymmetric cell divisions, in which daughter cells often inherit different sets of protein components from their progenitors, and consequently develop different properties. Such activity is highly coordinated, to ensure that the appropriate number and type of cells are produced at just the right times and locations. The Drosophila neuroblast, a type of stem cell, provides a good example of this, dividing perpendicular to its overlying epithelium to give rise to a pair of daughter cells of which one is a more highly differentiated intermediate neural progenitor and the other is a neuroblast like its parent cell. The means by which this division is oriented, however, are not well understood. Previous work has indicated that centrosomes and microtubules play a crucial role in larval brains, but other studies suggest that other factors may be important as well in embryonic central nervous systems.
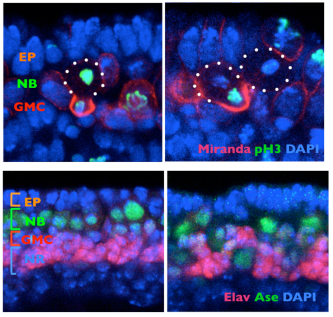
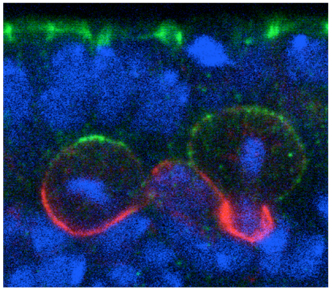
Shigeki Yoshiura and others in the Laboratory for Cell Asymmetry (Fumio Matsuzaki, Group Director) have now found that the key may lie in molecular signals across the cell membrane that tell neuroblasts how to align. Published in Developmental Cell, the group shows that the G protein-coupled receptor (GPCR) Tre1 is a key factor in a molecular interaction that orients these cells with respect to the epithelium. The group’s study began with a genetic screen for non-cell-autonomous factors involved in orientation, by monitoring the orientation of neuroblasts in embryos lacking genes coding for candidate membrane proteins. They found that mutation of the rhodopsin family orphan GPCR gene trapped in endoderm 1 (tre1) was associated with orientation defects, raising the possibility of a causal relationship. Deletion mutants for tre1 showed significant perturbations of neuroblast orientation with respect to the epithelial ectoderm, resulting in disorganized stratification of the developing tissue in central nervous system (CNS). This phenotype could be rescued by introducing a genomic fragment of the gene, strongly suggesting that it was responsible for the neuroblasts’ disorientation. Interestingly, however, cell polarity was unaffected by tre1 deletion, and the early-stage orientation defects in such mutants were rectified over the course of development by some unknown compensatory mechanism. Yoshiura et al. speculated that this correction might be due to interactions between neurons and other neighboring cells, such as glia. And indeed when they tested flies in which both tre1 and repo, a critical glial cell gene, were deleted, even later-stage neuronal organization was aberrant, suggesting that neuron-glia interactions are required for the development of the CNS. Using flies in which the distribution of Tre1 could be visualized, the group next sought to determine its mode of function. Yoshiura found that this protein was distributed widely in neuroblasts across the cell cortex, but to a much weaker degree in ganglion mother cells and neurons, indicating that it might function in a neuroblast-autonomous fashion. Disruption of microtubule activity had no effect on orientation in neuroblasts in the embryonic CNS in which Tre1 was normally expressed, suggesting that this protein functions independent of microtubules at embryonic stages.
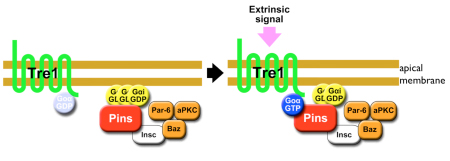
To exclude the possibility that Tre1 helps orient neuroblasts via signals from a tissue other than epithelium (such as mesoderm), the group examined a brain region in which only epithelium, neuroblasts and their progeny are present, and found that the cells remained oriented perpendicular to the epithelium, as expected. They next used co-immunoprecipitation to find binding partners of Tre1, and found that it interacts with GOα, a neuroblast protein of unknown function. Using a toxin that specifically interferes with GOα, they found that loss of this protein’s function results in a phenotype mirroring that of tre1 deletion, suggesting that Tre1 may orient neuroblasts in response to signals from the epithelium, by facilitating GDP-GTP hydrolysis by GOα. These findings brought another factor into the picture, as the protein Pins is known to interact with GOα in vitro. Pins is known for its role in mitotic spindle orientation, via binding with another Gα protein, Gαi. In vivo tests using a different assay, however, indicated that only the GOα-Pins interaction depends on Tre1, suggesting that only the GTP form (but not the GDP form) of Gαo binds to Pins. Additional testing using Gαi, in addition to Goα and Pins, in tissue cultures, showed that to be the case – the GTP form of GOα showed much higher interactivity with Pins than the GDP form of Goα or Gαi. Constructing a cell culture assay system, the group also found that Tre1 could recruit Pins in the presence of GOα. The scenario that emerges is a sort of “handoff” mechanism; binding with the GDP form of Gαi is necessary and sufficient for Pins to be recruited to the cell membrane. At the membrane, the GTP form of Goα, in turn, is generated from its GDP form only on the apical side proximal to the adjacent epithelium, from which an unidentified signal presumably activates Tre1. Gαi is then replaced by the GTP form of Goα, due to its more stable biding to Pins, which serves to recruit Pins to the apical membrane. In neuroblasts, Pins associates with the polarity-organizing aPKC-Par complex via interaction with Inscuteable, and consequently recruits this complex to the apical side of neuroblasts. As the aPKC-Par complex determines the polarity and orientation of division in neuroblasts, the Tre1-Pins mechanism ultimately directs the orientation of neuroblasts with respect to the epithelium. “While cell polarity in Drosophila neuroblasts is known to be organized in a cell-autonomous manner, our findings on the Tre1-Pins mechanism show that such self-organized cell polarity can be externally oriented,” says Matsuzaki. “This mechanism may be widely used in the orientation of asymmetric divisions or the polarity of cells relative to adjacent tissues, which may be important for directional tissue growth or stem cell environments.”
|
|||||||||
|
|||||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |