| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
June 10, 2012 – The cerebral cortex is the thin, but structurally complex, surface of the mammalian forebrain and serves as the locus for important functions of the nervous such as the processing of sensory inputs, decision-making, and memory. This “gray matter” shows a neatly stratified organization into six layers, each characterized by a distinct type of neuron, all of which connect and interact with other parts of the cortex, and the rest of the brain. Positioning of cells in their appropriate locations is therefore key to brain function. The mechanisms by which cortical neurons arrive at and establish themselves in their specific destinations, however, remain poorly understood. Yuko Gonda and others in the Laboratory for Neocortical Development (Carina Hanashima, Team Leader), working with colleagues at the National Institute of Neuroscience, have now shown how the receptor protein Robo1 helps determine the positioning of certain neurons in the neocortex. Published in the journal Cerebral Cortex, this work reveals an important new molecular basis underlying neuronal arrangement in the cortex.
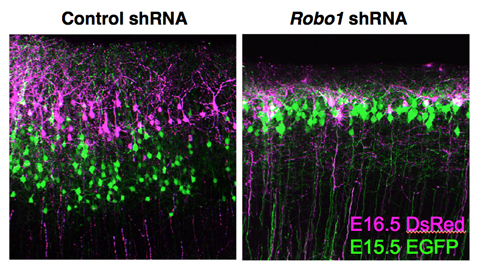
The Robo proteins were first identified as receptors for chemorepulsive signals in Drosophila, and were later found to operate in axon guidance in the mammalian central nervous system as well. In a screen to identify genes expressed in subset of neocortical neurons, Gonda first found that Robo1 mRNA exhibit layer-specific expression. Further analyses revealed the Robo1+ cells to be pyramidal neurons, and that the protein localized mainly in dendrites and axons. To better understand its function, the team first examined mice in which Robo1 had been deleted from the genome. They found higher densities of pyramidal cells in cortical layers II/III in these knockout mice, resulting in a thinning of these layers and consequent shift of layer IV toward the pial surface of the cortex. To further determine the cell-autonomous requirement for Robo1 in these cells, Gonda introduced shRNA constructs against Robo1 in discrete population of layers II/III pyramidal neurons by electroporation, and found that greater numbers of these cells were retained in the intermediate zone as opposed to entering the cortical plate as in controls, suggesting that loss of Robo1 function affects early neuronal migration in the embryonic cortex. The laminar organization of cortical neurons follows an inside-out pattern, in which the early-born neurons migrate to deeper layers of the cortex, while later-born generations make their way successively to more superficial layers. In Robo1 shRNA transfected layers II/III pyramidal neurons, although the cell movement into the cortical plate was initially delayed, these neurons eventually migrated to the surface of the cortical plate. However, after arrival, these neurons remained in the most superficial part of layers II/III. As the molecular mechanism by which the relative inside-out positioning of cortical neurons is established remains largely unknown, Gonda next assessed the distribution pattern of temporal cohorts of Robo1 suppressed neurons by performing sequential electroporation analysis. The experiment revealed that Robo1 suppression leads to abnormal laminar distribution of neurons in which the inside-out pattern was compromised. Interference with Robo1 function also caused defects in the growth and branching of neurites, while leaving neuronal identity intact. The picture that emerges is one in which Robo1 is required for determining the layer-specific final positioning of pyramidal neurons, and fine-tuning of axon projections in distinct cortical layers. “The laminar organization of the mammalian cortex is its most distinguishing feature, but we only have a limited understanding of the molecular factors that give rise to this structure,” says Hanashima. “What we have shown here is how a layer-specific axonal guidance receptor regulates the arrangement of neurons in upper layers of the cortex, which suggests that other molecular control systems may be at work in determining neuronal location in other layers as well.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |