| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
September 5, 2012 –Birds, many species of which experience rapid changes in air pressure during flight, possess a specialized structure called the paratympanic organ (PTO) in the middle ear that appears to function as a kind of barometer or altimeter. This fluid-filled sac is connected to the tympanum (ear drum) by a ligament-like tissue, by which minute pressure-induced changes in the membrane are communicated to the PTO, triggering deformations that stimulate mechanosensory hair cells lining its inner walls. The PTO is for the most part a birds-only structure, and is not present in mammals, amphibians, or most reptiles. The developmental origins of this organ, however, remain poorly understood. Using the chick embryo as a model, Paul O’Neill and others in the Laboratory for Sensory Development (Raj Ladher, Team leader) have now found that the PTO arises from a newly discovered tissue primordium known as a placode, and reconfirm its homology to a sensory organ in fish known as the spiracular organ. This work, published in Nature Communications, was done in collaboration with Clare Baker from Cambridge University, and resolves a long-standing question over the embryonic and evolutionary roots of this sense organ.
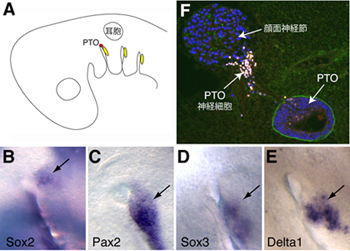
Previous studies have suggested that the PTO and spiracular organ (a sensory pouch found in fish thought to detect jaw movements) are homologous structures, based on similarities in tissue morphology, anatomical position, innervation, and the presence of mechanosensory hair cells. The case for this homology was called into question, however, by fate mapping experiments in chick embryos conducted in the 1980s that showed that the geniculate placode, which gives rise to facial neurons innervating the tongue, is the embryonic source tissue for the PTO. As the spiracular organ arises from a different placode, this made the homology between the two organs seem a less likely prospect, and for years the evolutionary relationship between them has been disputed. But O’Neill doubted that a placode that in other species gives rise solely to neurons would also generate the hair cells of the PTO, so decided to study its development in greater detail to get to the bottom of the question. The gene Sox2 is known to play a critical role in hair cell development, so he first examined its expression pattern at embryonic stages when the PTO begins to form, and observed a small patch of Sox2 expression immediately adjacent to the geniculate placode. Interestingly, the Sox2+ region did not express geniculate placode markers, suggesting that it represented a distinct entity. O’Neill next traced the fate of cells derived from the region and found that it gave rise to both the PTO and a population of PTO neurons which migrate to the geniculate ganglion. These findings pointed to a dramatic conclusion – that the PTO is not derived from the geniculate placode, but rather a previously undiscovered separate placode directly alongside it, which the team has christened the PTO placode. Interestingly, although neurons from the geniculate and this newly described placode both reside within the geniculate ganglion, they remain segregated by gene expression and the regions of the brain to which they project. To confirm that the PTO placode and geniculate placode are separate entities, the team next performed experiments in which different regions of head ectoderm were transplanted from quail embryos to the PTO precursor region of chick embryos to evaluate the developmental effects. When equivalent quail tissue was grafted into chick, both the PTO and the geniculate ganglion formed, but when quail tissue from different regions of the head or trunk was transplanted, the PTO failed to form, while the geniculate ganglion developed normally, suggesting that the two structures are formed by different developmental mechanisms. The discovery of a novel placode is an exciting finding by any standards, and in this case one that helps quell a decades-old controversy over the embryonic and evolutionary origins of the PTO. “The next step is to identity the key molecular signals underpinning PTO and spiracular organ formation, and hopefully to understand the evolutionary mechanisms responsible for PTO loss or retention in particular species” commented O’Neill. |
||||
|
||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |