| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Oct 10, 2012 –As is true of many brain structures, the hippocampus, which plays a central role in memory and learning, is made up of multiple cell types organized into domains controlling different functions. Distinct from many other brain regions, however, the hippocampus is a site of active neuronal generation in the adult. Its distinctive pyramidal neurons are contained mainly in the cornu ammonis (CA), while another hippocampal region called the dentate gyrus (DG) is characterized by granule cells. While it is known that the survival of these granule cells relies on the activity of specific transcription factors, how they arise during the differentiation of the hippocampus has remained unknown. In a recent study, Tomohiko Iwano and others in the Laboratory for Cell Asymmetry (Fumio Matsuzaki, Group Director) have now shown that the transcription factor Prox1 specifies the identity of postmitotic granule cells in mouse, while steering them away from a pyramidal fate. Published in Development, this work reveals how this single factor contributes to generating the cellular diversity of the hippocampus.
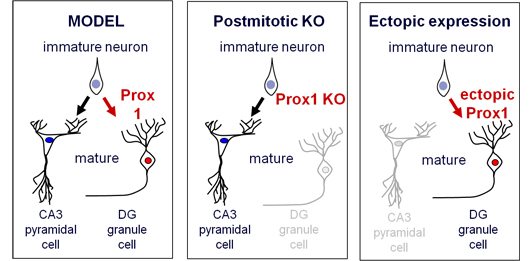
For neurons, granule cells are tiny but they play a major role in diverse brain regions, including memory formation in the dentate gyrus. Taking a lead from previous studies that showed Prox1 to be involved in maintaining granule cell survival, Iwano sought to gain a better understanding of whether this transcription factor might play a role in the differentiation of these neurons as well. As the Prox1 gene is constantly switched on in granule cells, the group first generated conditional knockouts allowing them to selectively switch it off at any stage in development. When they looked at DG neurons in which Prox1 was homozygously deleted in the postmitotic stage, the prospective granule cells lost the expression of all markers of both mature and immature neurons of that type. Even more interestingly, however, these conditional Prox1 mutant cells in the DG began to exhibit gene expression patterns and morphology reminiscent of pyramidal cells, the typical neuronal subtype found in a specific CA subregion of the hippocampus – a remarkable transformation for cells which might otherwise be thought of as terminally differentiated. Iwano found that granule cells exhibited similar expression levels of at least 18 marker genes characteristic of CA3 pyramidal neurons after postmitotic knockout of Prox1, and began to show typical pyramidal dendrite morphology as well. By looking at the effects of loss of Prox1 function at various stages in hippocampal development (which occurs mainly from late embryonic to neonatal stages of the animal, although granule cells continue to be generated throughout life in the DG), the group was able to show that at an advanced stage of hippocampal development and even in adulthood, Prox1 knockout was able to convert incipient granule cells to pyramidal-like neurons. A key aspect of a neuron’s function is its connectivity with other parts of the brain, both by accepting input and by projecting its axon to a specific site. Granule cells and pyramidal neurons ordinarily project to different destinations, so Iwano next checked whether Prox1-deficient DG cells would mimic not only pyramidal cells’ gene expression and morphology, but their circuit-forming behavior as well. He found that while some of the Prox1-homozygous granule cells retained their characteristic axonal destinations, the range of projection was greatly expanded to include sites typical of those of CA3 pyramidal neurons. Having determined the effects of Prox1 depletion, the group next tried the converse experiment to observe how its overexpression would affect hippocampal cell fate. Iwano watched primary cultures of cells electroporated with a Prox1 plasmid, they exhibited a much stronger tendency to gene expression and morphology typical of granule cells, as opposed to control cultures in which the pyramidal fate predominated, suggesting that Prox1 overexpression promotes granule cell fate at the expense of pyramidal. Electroporation of the same plasmid in vivo had equally pronounced effects in inducing the granule cell phenotype. Thus, the group indicated that both depletion and acquisition of Prox1 expression at postmitotic hippocampal cells could induce a conversion of these cells’ neuronal fate (from granule cell to pyramidal, and from pyramidal to granule, respectively), demonstrating that Prox1 is a kind of on-off switch for pyramidal vs. granule neurons, even in at advanced stages of neuronal maturation “It’s been thought that pyramidal neurons and granule cells in the hippocampus are of totally different origin, but our finding reveals that their destinies are not so securely fixed, at least not until right before their maturation by the integration into the circuit, which takes about three weeks after their birth,” says Matsuzaki. “This may be a property common to other neurons as well, which ensures the flexibility and tunability of neural circuit formation during both brain development and adult neurogenesis.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |