| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
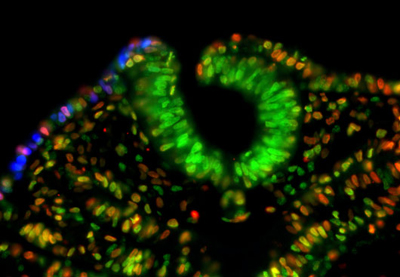
November 16, 2012 –Thickenings in ectodermal tissue in the early embryo, known as placodes, serve as the developmental starting points for a great many neural and sensory tissues. The region that gives rise to the inner ear, known as the otic placode, and the epibranchial placode, which generates craniofacial neurons, are induced by the FGF signaling pathway acting on a shared domain called the posterior placodal area (PPA). Studies in a variety of model organisms have suggested that the Pax family of genes may also function in the formation of these placodes, or the PPA as a whole, but the evidence has remained equivocal. Now, Sabine Freter and colleagues in the Laboratory for Sensory Development (Raj Ladher, Team Leader) in collaboration with the Genome Resource and Analysis Unit have shown that Pax2 functions not as an inducer, but a regulator of cell proliferation, in the otic and epibranchial placodes. Published in Developmental Dynamics, the report adds a new twist to our understanding of Pax family genes in tissue maintenance.
Early work in mouse, zebrafish, and frog indicated that the gene Pax8 is expressed earlier than Pax2 in the nascent inner ear, and it was thought that either or both might play an inductive role in otic placode or PPA formation. The understanding of the function of these Pax genes was muddled somewhat when subsequent studies revealed that Pax8 mutants showed no inner ear phenotype, and to add to the confusion, analyses of chick development revealed that loss of Pax2 function had no effect on induction of the PPA. Seeking to get to the bottom of the question, Freter sought to study the role of Pax2/8 in chicken otic development. Interestingly, after attempts to clone the Pax8 gene and to identify it through searches of the chick genome, the team was unable to identify the orthologous sequence in chick, or in the genomes of other related species, suggesting it had been evolutionarily lost. Pax2, however, is highly conserved, indicating that it is likely the sole Pax gene functioning in PPA development in birds. Freter next used RNAi to interfere with Pax2 function, but found that this had no effect on PPA formation. Similarly, overexpression of the gene also left the PPA apparently unaffected. Suspecting that the true role of Pax2 might be in inner ear differentiation rather than induction, the team looed at a later stage in development, when inner ear-specific genes normally begin to be expressed. Pax2 knockdown resulted in the complete loss of expression of Soho1, an early marker of inner ear, but did not appreciably affect the patterning of the otocyst, but did cause a reduction in size. Its overexpression, in contrast, inhibited inner ear differentiation. The epibranchial placode, which contributes to various cranial ganglia, also derives from the PPA, so Freter investigated whether Pax2 inhibition would affect its formation or differentiation as well. She found that, as in the otic placode, repression of Pax2 function had no effect on the expression of genes associated with the induction of this placode, but dramatically reduced expression of genetic markers of committed neuronal progenitors. And, as in the inner ear, overexpression of Pax2 interfered with epibranchial differentiation as well. Seeking to better understand the mechanism by which Pax2 affects the otocyst and epibranchial tissue, the team next investigated the possibilities that its effect could be due to altered cell death or differentiation. The absence of caspase upregulation following Pax2 knockdown suggested that an effect on programmed cell death could be ruled out. The cell cycle, however, was slowed significantly by Pax2 inhibition, causing an overall reduction of mitotic activity in affected cells. Interestingly, overexpression of Pax2 had no appreciable effect on cell cycle. “Given that Pax is known to work in the maintenance of precursor cells in other tissues, such as skeletal muscle, these latest results point to the possibility that Pax plays a general role in cell division and the maintenance of an undifferentiated state,” says Ladher. |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |