| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Jul 13, 2013 Uniquely among sensory tissues, olfactory neurons have the capacity to regenerate throughout life, a necessity given their direct exposure to the external environment. The olfactory neuroepithelium itself derives from a sensory placode includes a variety of supporting cell types in addition to neuronal cells, which are generated by the activity of progenitors also present in the tissue. Despite our increasingly detailed understanding of olfactory neural circuitry and functions, however, we still have much to learn about the ontogeny and regeneration of olfactory neurons. Work by Marie Paschaki and colleagues in the Lab for Sensory Development (Raj Ladher, Team Leader) published in Neural Development has now begun to provide some insights into those processes. By studying the function of retinoic acid in olfactory tissues from mouse and chicken, Paschaki et al. show that this signaling molecule plays a role in olfactory neurogenesis.
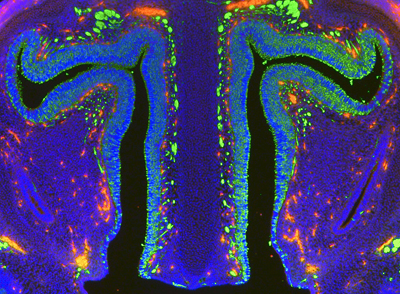
Retinoic acid (RA) is involved in a broad range of developmental processes, and had been implicated as a possible signal involved in olfactory epithelial patterning. But the results of this previous work had left open the question of whether it was involved only in induction of the tissue, or in prompting neuronal differentiation as well. Paschaki set out to pinpoint this role, as well as to determine the extent (if any) to which retinoic acid affects olfactory neurogenesis, by studying RA and an enzyme responsible for its synthesis, RALDH, in chicken and mouse. She first examined whether inhibition of RA signaling would perturb the induction of the olfactory placode in chicken embryos, but found that interference with RA synthesis had no perceptible effect. In mouse mutants lacking Raldh function as well, showed no abnormal placode phenotype, indicating that retinoic acid is dispensable in olfactory placode induction. That picture changed dramatically however when she tested RA function later in development. When she implanted beads soaked in an inhibitor of RALDH activity into chicken embryos, she found that this led to a failure in the differentiation of olfactory stem cells, suggesting that RA is necessary for inducing or maintaining the olfactory progenitor population. In tissue explants, culture medium containing vitamin A, from which retinoic acid is metabolized, revealed an inhibitory effect on neurogenesis, pointing to the possibility that RA maintains stem cells by suppressing differentiation. These observations raised the issue of where in the olfactory lineage RA plays its part. Loss of RA synthesis had no affect on apoptosis, but did increase the number of proliferating cells, prompting Paschaki to look at the olfactory progenitor phenotype in a mouse Raldh mutant. She found that a specific population of quiescent Pax6-expressing progenitors was depleted in the mutant embryos. Turning again to explanted tissue, she examined the effects of vitamin A and found that its addition to the culture medium supported greater numbers of progenitors than did medium without this RA precursor. More strikingly when she grew Raldh null mutant explants in the same conditions, the progenitor population collapsed irrespective of whether vitamin A was included in the medium, suggesting that massive differentiation had occurred at the expense of progenitor self-renewal. This hypothesis was borne out in the mutant embryos, which showed fewer Pax6-expressing progenitors than in wildtype, and consequent failure to maintain olfactory neurogenesis. "The olfactory epithelium is the only neuronal population in mammals that can repair itself, says Ladher. If we can understand how it uses its developmental history to achieve this, it may give us some ideas on how we can help other neuronal populations repair themselves. |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |