| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Jun 7, 2013 – While body sizes may differ significantly between individuals within the same species, body shape tends to remain constant. Even in closely related species, such as mouse and rat, while the overall size of the individual animals is quite different, the shape of their bodies are very similar. In a more extreme demonstration of this phenomenon, it has been shown that, after being cut in two, the dorsal half of a frog embryo can develop into a smaller, but morphologically ordinary tadpole, rather than one featuring only dorsal structures. The reason behind this conservation of pattern in differently sized embryos, however, has remained a mystery. A new study by Hidehiko Inomata and colleagues in the Lab for Organogenesis and Neurogenesis (Yoshiki Sasai, Group Director), in collaboration with the Lab for Physical Biology (Tatsuo Shibata, Research Unit Leader), now points to a role for gradients of signaling factors from the embryonic organizer region of the African clawed frog, Xenopus laevis, in maintaining proportional patterning. Published in Cell, these new findings stand to answer a longstanding question of how embryonic shape can be scaled to embryo size.
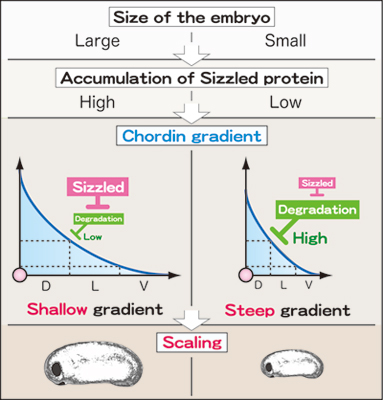
In early amphibian development, BMP signals from the ventral side of the embryo are countered by BMP antagonists secreted from the organizer regions, first described by Hans Spemann in the 1920s. The interaction between these two streams generate a dorsal–ventral signaling gradient, which in turn sets the stage for further developmental routines: neural lineages form in dorsal regions of higher organizer signaling activity, while lower activity at the ventral side of the embryo triggers differentiation into blood and other lineages. What has remained an enigma is that that some mechanism must enable the maintenance of this gradient, even when the size of the embryo is halved, as suggested by the bisection studies described above. To dig deeper into this question, Inomata first developed a ‘hyperventralized’ embryo to allow him to zero in on and analyze the functions of discretely expressed genes in reconstructing the dorsal–ventral (D–V) axis. When he misexpressed the organizer signal Chordin, he found that a gradient akin to that seen in wildtype embryos was established, leading to the formation of a normal D–V axis. Gradients are defined by distribution, so Inomata was curious about what factors that control how Chordin is distributed in the embryo, and discovered that the action of Sizzled, which inhibits its degradation, plays a key role in stabilizing the Chordin molecule along a grade. Specifically, when he changed the level of Sizzled expression, he found that the Chordin gradient was altered dramatically, tilting from a gradual slope to a steep cliff. Inomata determined that the interaction between these two factors involved the following feedback loop: stabilization by Sizzled enables the broader diffusion of Chordin, which Chordin itself limits by inhibiting further Sizzled expression. What then of dorsalized embryos? Knowing their ability to grow into small but proportional larvae, Inomata examined gene expression in bisected embryos, and found that the expression levels of dorsal markers in dorsal halves also dropped by around 50%, even as overall shape was maintained. But in loss-of-Sizzled-function embryos, bisection triggered no such reduction in dorsal gene expression, and the embryos failed to develop normally, suggesting that Sizzled is responsible for maintaining the Chordin gradient in a manner scaled to embryo size. The group’s conclusion, that stabilization of Chordin by Sizzled and inhibition of Sizzled by Chordin maintain the Chordin gradient in a size-dependent manner, gained further support from the results of simulations of various starting values and perturbations for these factors and embryo size. “It will be interesting to discover whether a similarly balanced feedback mechanism is at work in the embryos of other species,” says Sasai, “as well as to determine whether the scaling mechanism changes over time as the embryo grows.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |