| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Jun 21, 2013 – British developmental biologist Lewis Wolpert famously remarked that the most important events in one’s life take place during gastrulation. This crucial stage of embryogenesis involves dynamic morphological rearrangements that give rise to the three germ layers of the body: ectoderm, mesoderm, and endoderm. While gastrulation as a general phenomenon is highly conserved, the details differ between major phylogenetic groups. In fish and amphibians (anamniotes), for example, the induction of mesoderm is centered on a site known as the blastopore, while in amniotes such as birds and mammals, mesoderm forms at the primitive streak. Despite the central importance of this process, however, the evolutionary roots for this diversity in its developmental program have remained elusive. Cantas Alev and colleagues in the Lab for Early Embryogenesis (Guojun Sheng, Team Leader) have now showed that, with a little manipulation, chicken embryos can be made to undergo an anamniote-like form of mesoderm induction. Published in Development, these results shed new light into the evolution of gastrulation strategies.
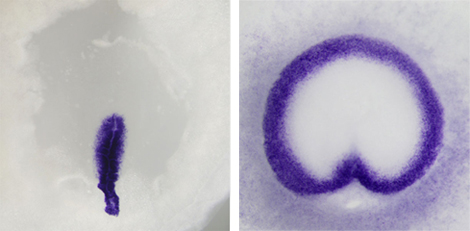
In frog and other anamniote embryos, gastrulation proceeds radially outward from the blastopore, inducing a ring of cells that migrate to the embryo interior, forming the mesodermal layer. In amniotes such as chicken, however, mesoderm cells are formed solely from a region known as the primitive streak, which evolved as an alternative to the ‘circumblastoporal’ routine. Despite these differences, both forms of gastrulation involve the expression of conserved signaling factors, such as FGF, Wnt, and TGFβ. What Alev sought to determine in the present study was whether he could recapitulate the more ancient, anamniote approach to gastrulation in an amniote embryo by tweaking these signaling pathways. He first developed a new method for injecting molecular signals into the subgerminal cavity of the developing embryo, and used it to test the effects of widespread ectopic presence of prominent signaling factors. He found that injection of FGF4 induced a ring of mesoderm (as labeled by Brachyury) in the marginal zone, irrespective of whether the primitive streak formed or not (the streak formed in fewer than half of the injected embryos). The mesoderm thus formed expressed epithelial–mesenchymal transition (EMT) markers, as in normal chick gastrulation, and dorsal–ventral axis formation was unperturbed, suggesting that these bird embryos could be led to undergo a very frog-like form of gastrulation, independent of primitive streak formation. In these manipulated embryos, FGF presence was not able to induce mesoderm in the central epiblast, raising the likelihood that other factors important for mesoderm formation were also involved. When Alev co-electroporated Wnt and FGF signaling factors into the medial epiblast of chick embryos, he found that the combined activity resulted in induction of mesoderm even in this central region. Additional experiments modulating a third signaling molecule, TGFβ, revealed that this pathway plays roles in the dorsalization of the embryo as well as the ingression of mesoderm cells. Suspecting that the effects of FGF activation might be conserved in amniotes other than chick, Alev repeated the experiment in quail, emu, and turtle embryos, and observed similar reversion to an anamniote-like circumblastoporal mode of gastrulation. “Seeing dramatic differences in the morphogenetic processes taking place during gastrulation between anamniotes and amniotes, one would expect that this would also entail a significant change in the underlying molecular mechanisms” says Sheng. “But the results from these new experiments clearly show that, whether in frog or in bird, the essential process is fundamentally similar.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |