| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
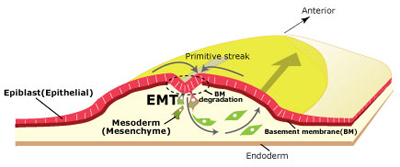
Sep 18, 2013 – In higher animals, gastrulation lays the groundwork for subsequent development by establishing the three germ layers that give rise to all the tissue in the body. In chicken and other birds, cells of the epiblast at the body surface migrate to a site called the primitive streak, and subsequently move inward to the embryonic interior, forming the endoderm and mesoderm. Epiblast cells are epithelial in nature, form adhesive bonds and attach to a basement membrane. But during their ingression from the primitive streak, these cells shed their connections with other cells and the basement membrane, transforming into mobile mesenchymal cells in the process. Such epithelial–mesenchymal transitions (EMT) play roles in numerous developmental processes, but the molecular machinery that controls EMT is still poorly understood. Yukiko Nakaya and colleagues in the Lab for Early Embryogenesis (Guojun Sheng, Team Leader) have now identified once such mechanism, finding that the microtubule-binding protein CLASP is required for maintaining cells in an epithelial state, and that downregulation of CLASP induces the degradation of basement membrane, a necessary event in gastrulation. Published in Journal of Cell Biology, this work was featured on the cover of the August issue.
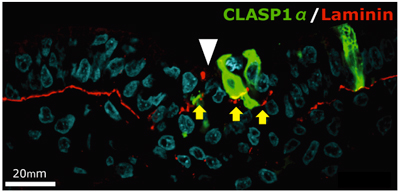
Previous studies of CLASP had shown that it associates with microtubule plus-ends and maintain their density, but the physiological and developmental role of the protein has been a mystery. Nakaya had observed that EMT in gastrulating chick embryos appeared to involve microtubule-mediated anchoring of epiblast cells to the basement membrane, presumably linked to its maintenance and degradation. Looking first at the expression of CLASP during this period, she observed that while it was expressed in the epiblast, it was downregulated in cells at the primitive streak. Overexpression of CLASP caused stabilization of the basement membrane, which is ordinarily destined to decay during EMT, while conversely, knocked down CLASP outside the streak led to abnormal degradation of basement membrane components. Investigating CLASP function, Nakaya found that it works with its binding partner LL5 to tether microtubules to the basal cortex in cells, suggesting that its expression maintains epiblast cells in an epithelial state, while its downregulation is a necessary step in EMT. But how does a cytoplasmic molecule such as CLASP have this effect on the extracellular basement membrane? For a promising candidate, Nakaya next turned to Dystroglycan, a transmembrane protein known to interact both with the basement membrane and other intracellular factors. Overexpression or inhibition of Dystroglycan in the epiblast yielded similar results to those she observed in her experiments with CLASP, inappropriate stabilization or degradation of the basement membrane. Co-immunoprecipitation revealed colocalization of the two proteins in the basal cell cortex, indicating a physical interaction. And when she looked at Dystroglycan expression in the primitive streak, she found it decreased during EMT, just as with CLASP. This series of experiments makes a solid case for the combined roles of CLASP and Dystroglycan in anchoring microtubules to the basal cell cortex and stabilization of the basement membrane in epiblast cells. “But we are still curious about why it is that loss of CLASP causes the basement membrane to decay,” says Sheng. “We suspect this may involve vesicle transport by microtubules, but we will have to study that possibility more closely in a future project.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |