| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Sep 24, 2013 – All the many odors we recognize and remember are first detected as molecular signals by G-protein coupled receptors (GPCRs) on specialized neurons in the olfactory system. The mouse has around 1000 such odorant receptors, which, when bound to an odorant molecule, convey signals to the olfactory bulb in the brain. An equal number of structures, known as glomeruli, serve as convergence points for neurons bearing the same odorant receptor, patterns of the collective activity of which are perceived as olfactory sensations by the brain. Establishing the right connections from receptor to glomerulus requires the precise wiring of olfactory neurons into elaborate circuits, by processes that remain imperfectly understood. Takeshi Imai, Team Leader of the Lab for Sensory Circuit Formation, and colleagues have now discovered an essential role for the activation of odorant receptors unprovoked by ligand binding in the control of axon targeting from olfactory neuron to glomerulus. Reporting in Cell, they also describe how the co-action of the protein Gs supports the efficient transduction of such weak, unstable signals, making guided axon projection in the fetal mouse possible even in the absence of odor stimuli.
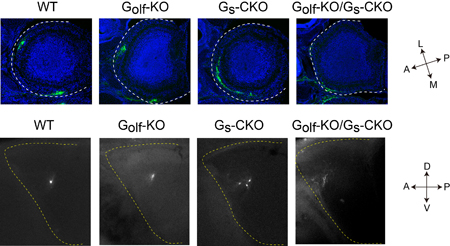
Odorant receptors are expressed on neurons lining the olfactory epithelium, each of which typically expresses only a single receptor type. Axons from these neurons of the same type converge to glomeruli specific to that odorant receptor profile, following a general targeting gradient along the anterior-posterior (A-P) axis, followed by more precise local sorting. Both of these have been thought to rely on signals arising from odorant receptor activation, but the details of these mechanisms have eluded explication. Binding of a GPCR by an odorant molecule triggers a shift from inactive to active state that entails structural changes in the conformation of the receptor molecule. Past studies have shown, however, that activation by such an agonist is not necessary; GPCRs show a certain baseline level of activity even in the absence of ligand binding, although this has often been considered little more than noise. Imai and colleagues set out to determine whether this agonist-independent receptor activation plays a role in olfactory neuron axon guidance along the A-P axis by developing mutant mice with altered baseline activity. A-P axon guidance is governed by factors during mid-development, while those responsible for local sorting are expressed later. In an extensive screening study, Imai was able to identify Gs as the A-P factor and Golf as the glomerular segregation molecule. Both proteins are structurally similar, and mediate odorant receptor cAMP signals; Golf is known to cooperate with odorant receptors in detection of odor stimuli but the role of Gs and how it works with Golf had never been determined. Undertaking a detailed biochemical analysis, Imai found that while Gs effectively responded to baseline OR activity, Golf did not. The team next generated knockout Golf and Gs mice; the Gs KO was necessarily conditional, as the mutant is embryonic lethal. Imai found that A-P axon guidance was dramatically affected in the Gs mutant, and glomerular sorting in the Golf KO, and that these defects were specific to the respective mutations. Gs mutants additionally showed a high frequency of axonal wiring errors, indicating that its role is to regulate A-P axon targeting in response to baseline signaling by still-developing olfactory neurons. “The ability odorant receptors to achieve two highly distinct functions simply by the use of differential responses by Gs and Golf to baseline signals is truly amazing,” says Imai. “Many have suggested that stimulus-independent signaling might play a role in neural circuit formation, and it appears that is just what we are observing here. We next want to study the mechanism behind how connection specificity during embryonic neural circuit formation depends on neuronal activity.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |