| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Nov 25, 2013 – The neurons of the enteric nervous system undergo among one of the longest journeys in development, as precursors migrate downward and proliferate to innervate the digestive tract from end to end. This process involves two main stages: primary caudal migration by neural crest-derived precursors, and a second round of radial migration of a subset of precursors that radiate to the submucosal layer. While precursors in the myenteric ganglia (MG) typically leave mitosis by the time of birth, many submucosal ganglia (SMG) precursors remain active for two weeks thereafter, suggesting that these cells at least in part remain in an incompletely differentiated state. The molecular controls over this exquisitely coordinated activity, however, have remained elusive. Toshihiro Uesaka and colleagues in the Lab for Neuronal Differentiation and Regeneration (Hideki Enomoto, Team Leader) now reveal that GDNF signaling is an essential regulator of both the primary and secondary migration of ENS precursors in mouse. Published in The Journal of Neuroscience, these new findings also show that intracellular signaling levels controlled by GDNF regulate differentiation states in these precursors as well.
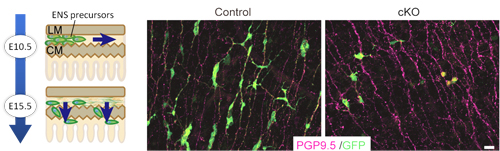
At embryonic day 9.5 in mouse embryonic development, neural crest-derived cells begin to enter the foregut and migrate down the enteric tract toward the colon, completing this primary migration by embryonic day 14. Most of this first wave of migrants differentiates to form myenteric ganglia, but a subpopulation secondarily migrates radially into the submucosal layer, giving rise to submucosal ganglia. GDNF is expressed in the gut mesenchyme, and activates the RET tyrosine kinase through binding with the receptor GFRα1. All of these factors are essential for proper ENS development, as evidenced by the loss of enteric neurons in mice lacking any of these genes. Previous work by the Enomoto lab has shown that enteric precursors are receptive to GDNF signaling into the postnatal period, suggesting that this factor plays a long-term central role in controlling multiple events during ENS development. Uesaka used a number of conditional knockout mouse lines to gain a more detailed understanding of GDNF’s function. When he examined GFRα1 expression, he found that in both myenteric and submucosal ganglia, GFRα1+ cells (i.e., those carrying receptors for GDNF) also expressed Sox10, a marker of undifferentiated cells. Knocking out GFRα1 at day 10.5, he found that mutant precursor cells lost most of the migratory activity seen in control ENS precursors. Knockout of the same gene at day 15.5, during secondary migration, caused a near-complete failure of submucosal ganglia formation. These results indicated that not only does GDNF signaling play important roles throughout ENS development, but that its activity changes over time. Looking at temporal changes in GDNF expression, the team found that while GDNF was highly expressed in both the longitudinal and circular myenteric layers, by day 18.5, when MG formation was complete, its expression was confined mainly to the circular muscle layer, highlighting the possibility that this shift in the site of GDNF expression might be responsible for inducing secondary migration. The formation of submucosal ganglia subsequent to MG formation indicates that even during their differentiation, the MG population must retain some undifferentiated cells. Here again, the team found that differential GDNF expression plays a role, as shown by the higher levels of a GDNF downstream factor in differentiated enteric neurons, and lower levels in undifferentiated precursors at day 12.5. Uesaka tested this hypothetical role for GDNF signaling in controlling cell state by knocking out RET, which interrupts GDNF signaling. Whereas most precursors would have differentiated into neurons within a few days, he found that in the RET knockout many remained undifferentiated. Recovery of GDNF signaling rescued the defects in migration and differentiation, sealing the case for its broad functionality. “This work presents a very reasonable system for the long-term maintenance of precursors in an undifferentiated state, and show why even seemingly minor changes in GDNF signaling receptors can lead to major developmental defects,” says Enomoto. “At the same time, we know that many other factors are also involved in regulating ENS development, so we will be curious to learn more about these are orchestrated in the embryonic gut.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |