| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
| Dendritic spine dynamics | ||
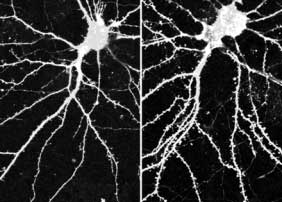
April 15, 2004 - Neural networks are made up of billions of interconnected axons and dendrites, neuronal projections that send and receive messages across junctional sites known as synapses. A synapse is formed when an axon (the projection that conveys outgoing signals) from one neuron establishes a stable junction with a dendrite (a signal-receiving neuronal branch) from another, allowing electrochemical messages to be exchanged. Dendrites are themselves covered with even smaller projections, called spines, which extend and make contact with other nerve cells. These dendritic spines are dynamic structures that are continuously projecting from and retreating back into the dendritic stem, stabilizing only on contact with another neuron under the right conditions . A report by Kentaro Abe and Masatoshi Takeichi of the RIKEN Center for Developmental Biology
(CDB; Kobe, Japan) and colleagues now shows that this synapse-forming dynamic is regulated by
the activity of the αN-catenin molecule. Catenins are already known as key players in cell-cell
adhesion, linking the cytoplasmic tails of cadherin transmembrane proteins to the actin cytoskeleton.
This new study, published in the April issue of Nature Neuroscience , shows that αN-catenin,
a form of catenin specific to the nervous system in mouse, also controls both the motility and
the stability of dendritic spines .
In many types of neurons, dendritic spines are formed when a dynamic fingerlike projection called a filopodium matures into a stable mushroom-headed structure, a process that was shown in a previous report by Takeichi et al. to rely on cadherin activity. In neurons from mice in which the gene for αN-catenin had been deleted, filopodia appeared and disappeared more frequently and protruded more dynamically than did those from normal mice, and failed to form stable synapses. When αN-catenin was overexpressed, however, the opposite was found: the overall density of spines increased and the mushroom caps in mature spines were slightly exaggerated, indicating that the rise in αN-catenin levels was linked to a concomitant increase in dendritic spine stability. Spine morphology is also known to play an important role in synaptic plasticity, the ability of synapses to respond to changes in neural activity. The team used two neuroactive agents - tetrodotoxin (TTX), a neural blocker known to convert spines to filopodia in hippocampal neurons, and bicuculline, a GABA inhibitor that increases neural activity - to study the effects of altered activity levels on αN-catenin. TTX was found to reduce the concentration of αN-catenin at synapses, but not its overall expression, suggesting that lower activity affected αN-catenin distribution. Conversely, in the bicuculline-treated cells, synaptic αN-catenin levels were intensified and expression was slightly higher. Curious about whether the morphological changes seen in TTX-treated spines were the cause or the effect of the lowered αN-catenin concentration, the group next tested the effect of tetrodotoxin on αN-catenin-overexpressing hippocampal neurons and found them to be unresponsive to the drug. This TTX-resistance was not seen on the overexpression of either N-cadherin or B-catenin (other important components of the neural cadherin-catenin complex), suggesting that αN-catenin acts as a primary mediator of neural activity-dependent changes in spine morphology, a process considered to be central to synaptic plasticity and the remodeling of neural circuits. These studies have provided strong evidence of αN-catenin's role in neural network stability
in vitro. Its physiological role, however, remains to be shown, as the lethality of the αN-catenin
knockout mutation has made in vivo studies problematic. The lab next plans to use an alternative
method that will allow them to bypass this lethality, and confirm their findings in live
animals, which promises to lead to a better understanding at a molecular level of how the brain
rewires itself in response to changes in neural activity. |
||
|
||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |