| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
| Fat binds the "bones" of the cytoskeleton | ||
May 26, 2004 - The cytoskeleton gives structure, strength and mobility to the body of the cell through a network of microscopic struts, cables and a lattice-like cortex. One of the principal components of this skeletal frame is a protein called actin, which is most prevalent in cellular peripheries where it provides a stable but regulable meshwork enabling the cell to maintain its structural integrity while responding to external and internal stimuli through shape alterations and movements. Cells receive such environmental signals via an array of pores, channels and membrane proteins, including the cadherin family of cell adhesion proteins, which (among other functions) allow cells to recognize and bind to other cells of like type. Now, in a study of these cell junctional regions, researchers at the RIKEN Center for Developmental Biology (CDB) have discovered new evidence of a direct link between cadherins and the actin cytoskeleton. The cadherins form a superfamily of proteins that includes the classic cadherins, known for
their function in cell-cell adhesion, and a number of other subfamilies with diverse activities.
The largest cadherins are molecules known as Fat cadherins, which feature a "tail" projecting
outward from the cell membrane that is nearly seven times the length of extracellular regions
in classic cadherins. It has been suggested that this extra length of the extracellular tail may
play a role in the regulation of the size of intercellular junctions in some tissues, such as
the renal glomerulus, in which the size of the gaps between specialized cells known as podocytes
has been linked to Fat cadherin activity.
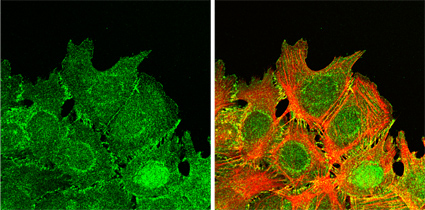
On the inner side of the cell membrane as well, the Fat cadherins (there are three mammalian subtypes of the Fat molecule) are nearly entirely different from the classic cadherins in terms of protein structure, a uniqueness that has prompted some scientists to postulate a divergent function for the Fats independent of the cell binding and recognition activities of the classic cadherins. Seeking to resolve the question of Fat function, Takuji Tanoue and Masatoshi Takeichi, working in the Laboratory of Cell Adhesion and Tissue Patterning (Masatoshi Takeichi, Group Director), focused on identifying the regions where mammalian Fat1 localized in cells, and found it to be present in filopodia, lamellipodia and cell-cell contact sites in certain types of cells. This distinct distribution at the dynamic edges of cells led Takeichi and Tanoue to investigate the possibility of a connection between Fat and the actin cytoskeleton, given the tight overlap between their patterns of expression. Knocking down Fat1 activity by RNA interference (RNAi), they found that Fat was required for the proper organization of actin structures in the region as well as for the initiation and maintenance of tight cell-cell binding. A separate assay revealed that Fat1 is also needed to establish cell polarity at the margins of wounds during the healing process; correct polarization is essential for cells, such as those in damaged tissue undergoing repair, that need to know which side is facing "out" and orient themselves accordingly. Analyses at the molecular level showed Fat1 to have three consensus sequences for a binding site on a second protein, Ena/VASP, known to regulate the activity of the actin cytoskeleton. Further tests using fusion proteins determined that Fat1 does indeed bind directly with Ena/VASP, while studies of a mutant form of Fat1 with amino acid substitutions in the Ena/VASP binding site confirmed the association between these molecular partners at the cellular level. Looking again at the peripheral zones where actin and Fat1 co-localize, Takeichi and Tanoue found that fat1 regulates actin dynamics through its interaction with Ena/VASP, as shown by dramatic decreases in the formation of actin stress fiber formation in cells expressing mutant versions of Fat1 lacking a functional Ena/VASP binding site. RNAi knockdown of Fat1 further demonstrated its importance in the formation of tight cell-cell associations; associations between cells in which Fat1 was knocked down were significantly looser than those in normal cells, and actin organization appeared severely disrupted. Taken together, Tanoue and Takeichi's work, published in the May 24 online issue of the Journal
of Cell Biology, provides compelling evidence for the action of a Fat cadherin on one of
the most extensively studied components of the cytoskeleton, shedding new light on the dynamics
of actin regulation and suggesting an intriguing new role for cell adhesion molecules. |
||
|
||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |