| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
| Breaking new ground in germ cell guidance | ||
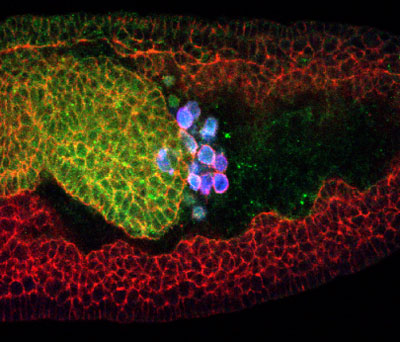
September 8, 2004 - Many types of cells are able to make their way through the body propelled by molecular motors and guided by pathfinding mechanisms. One of the great migratory phenomena in development is the journey undertaken by primordial germ cells in the Drosophila embryo. These cells form externally to the embryo at its posterior pole, are carried within by a process of invagination and navigate unerringly through the body to contribute to the formation of the gonad, acquiring the characteristics of more mature germ cells en route. The puzzle of how these cells find their way through the developing body has challenged biologists for years, but is now beginning to yield to their painstaking efforts toward identifying the underlying mechanisms that guide these travelers on their way. Kazuko Hanyu-Nakamura and colleagues in the RIKEN Center for Developmental Biology Laboratory for Germline Development (Akira Nakamura, Team Leader; Kobe, Japan) have fit a new piece into this jigsaw with findings recently published in the online edition of Development , describing an intriguing new model of germ cell migration involving a pair of guidance molecules, Wunen and Wunen2, and their discrete activities in germ and somatic cells.
The study began with a screen for mutant flies, which uncovered a phenotype in which the flies' primordial germ cells, called "pole cells," showed defects during their migration: the pole cells died in large numbers at a stage when they would normally begin to associate with the gonadal mesoderm. The genetic deficiency responsible for the defect was identified as a maternal effect mutation, meaning that its function (or loss of function) relies entirely on transmission by the mother fly. A closer analysis of the failing pole cells indicated that they began their development and migration normally, and that their deaths did not display a significant increase in the molecular activity most commonly associated with programmed cell death - activation of apoptosis mediator, caspase-3. Hanyu-Nakamura et al. pinpointed the site of the mutation to the locus for the guidance gene, wunen2 . The products of this gene and its relative, wunen , have been known for some time to play important roles in the guidance of Drosophila pole cell migration by somatic cells. The genes encode lipid phosphate phosphatases (LPPs), which it had been proposed degrade an extracellular substrate, thereby creating an environment unfavorable to germ cells and steering them toward their gonadal destination. The overexpression of either gene in somatic cells had also been shown to cause the death of pole cells, indicating their importance to germ cell viability. A maternal-effect function for wun2 , however, had never been reported, spurring the team to examine its role more closely. What they found surprised them. While wun and wun2 cause pole cell die-offs when overexpressed in somatic cells, the team found that maternal wun2 activity is actually required to sustain pole cells in a cell-autonomous manner: cells lacking maternal wun2 died in significantly greater numbers during migration. This suggested that wun2 had nearly opposite effects on germ cell survival depending upon whether it was expressed in somatic or primordial germ cells. Hanyu-Nakamura proposes that this may be caused by a situation in which zygotically expressed Wun and Wun2 in somatic cells and maternally supplied Wun2 in germ cells exist in a kind of competitive homeostasis, which might be the result of a shared uptake and dephosphorylation of a common substrate necessary for the survival of germ cells. In this model, too much Wun or Wun2 in somatic cells would deprive germ cells of the survival signal, causing them to die; too little Wun2 in the germ cells themselves would have the same effect. The putative substrate has yet to be identified, but if the Hanyu-Nakamura model can be validated, it may well represent a new paradigm for explaining the function of LPPs in developmental processes from axon growth and patterning to extraembryonic vasculogenesis.. |
||
|
||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |