| RIKEN Center for
Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
| How Tracheal Cells Know Where to Grow | ||
October 15, 2004 - The growing embryo is a hive of activity,
with cells stretching, wandering and assembling to form the higher-order
structures and networks that ultimately build the body. Some cells crawl
along a matrix, making their way to distant locations, while others, such
as some neurons, extend projections while the cell body remains in place.
These types of shape change and migration are acknowledged as fundamentally
important developmental phenomena, but scientists have long puzzled over
the guidance mechanisms that make sure that cells and their processes
end up in the right places. A number of migratory systems have been shown
to rely on molecules known as morphogens, which can act as either attractors
or repellants for migrating cells and steer them unerringly to their destinations. During its embryonic development, the fruit fly, Drosophila ,
assembles a trachea - a tubular respiratory network which delivers oxygen
to the rest of the body in the larva and adult. This organ arises from
ten pairs of tracheal placodes in thoracic and abdominal segments, which
send forth six primary branches that migrate in stereotypical patterns.
The dorsal branches move to points on the inner surface of the epidermis
on the medial axis of the embryo's dorsum (back) to fuse with their partner
from the opposite side. Each dorsal branch is tipped with a specialized
cell that leads the cells behind it, but the exact means by which these
terminal cells find their way across the interior face of the epidermis
to the dorsal midline has remained unknown.
In an effort to resolve this question published in the journal Development ,
Shigeo Hayashi (Group Director,
Laboratory for Morphogenetic Signaling) and colleagues at the RIKEN Center for Developmental Biology and the National
Institute of Genetics looked at molecules known to be involved in tracheal
branching for their potential roles as cell migration path determinants.
In order to study these molecular signals, Kagayaki Kato, a RIKEN special
postdoc toral fellow in Hayashi's lab, first tracked cell movements during
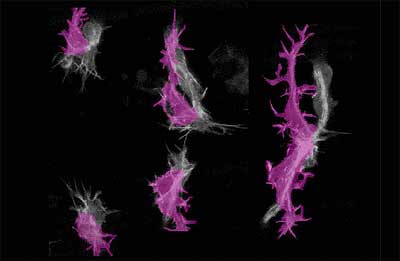
the process of tracheal development. Watching pairs of GFP-tagged cells found at the tip of the dorsal branch,
Kato saw that they migrated over the underside of the dorsal epidermis
(DE), where they made contact with partner cells from the opposite side
of the body. Throughout the process, these tip cells remained closely
associated with the epidermis, indicating that guidance signals might
be of epidermal origin. The team opted to focus on one of the tip cells,
called the terminal cell, which stretch es out, seemingly in response
to directional signals. At first, terminal cell filopodia project equally in all directions,
but only those which extend ventrally (toward the belly of the embryo)
stabilize; other filopodia tend to withdraw back into the cell body after
a short time. It is this stabilization that allows the terminal cell to
sprout its branch exclusively in a ventral direction. Hayashi et al. next
looked at the epidermal region immediately adjacent to the spot where
the dorsal branch tip cells congregate for specific patterns of gene expression
and noted that their migration and subsequent activity seemed to home
to a space underlying a dorsal epidermal region marked by the expression
of a pair of morphogens : Decapentaplegic (Dpp), and Hedgehog (Hh), which
are expressed in stripes in the DE. An experiment in which extra terminal
cells were generated supported the idea that these cells display a preference
for Hedgehog-positive zones, as terminal branch cells that were displaced
from one such region would make their way through non-Hh-expressing territory
to the closest Hh-positive segment. Suspecting a role for hedgehog in directing the outgrowth
of terminal branches, the team next made tests in which the gene was broadly
misexpressed or its signal transduction interfered with, and obtained
results that tended to confirm their hypothesis that external Hh influences
the direction of terminal branch outgrowth. Cells rendered unresponsive
to Hh signaling displayed aberrant dorsal branch migration, with filopodial
extensions radiating in all directions unrestricted to their usual ventral
orientation, indicating that tightly restricted expression of Hh is required
for normal terminal branch migration. Returning to the earliest stage of multidirectional outgrowth, the researchers
examined whether Dpp, which is expressed in the overlying dorsal epidermis,
might act as an inhibitor of terminal branch outgrowth, further ensuring
that foraying branches ultimately only travel downward to the Hedgehog
expressing regions. Dpp is already known to be important for dorsal branch
specification, so Hayashi and colleagues designed tests to investigate
whether it plays a specific role in branch migration. Experiments in which
Dpp was overexpressed showed that branches failed to extend as normal,
while Dpp downregulation resulted in misdirection of the terminal branch
along the anterior-posterior axis.
|
||
|
||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |