| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
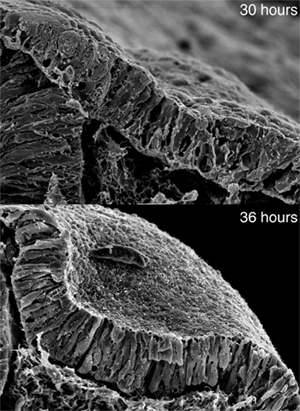
June 23, 2008 – The organs of the body are born from simple sheets of cells that later take on their elaborate and complex forms during development, a process known as morphogenesis. It is known that such developmental processes involve changes at the level of individual cells, but what influences groups of cells to collectively change shape in a coordinated fashion remains largely a mystery. The inner ear, which originates as a flat patch of epithelium that subsequently invaginates and pinches off to form a hollow cyst, provides an excellent system for the study of such organogenesis. In a new study published in Current Biology, XiaoRei Sai and Raj Ladher of the Laboratory for Sensory Development (Raj Ladher; Team Leader) have revealed the role of extrinsic signaling in the morphogenesis of the inner ear in the chicken embryo. Through a series of experiments involving chemical inhibition of signaling pathway components, they showed that, following a preliminary induction phase, FGF signaling from the underlying mesoderm instructs the cells of the ectoderm that are destined to become the inner ear to expand basally, contributing to the transformation of columnar epithelial cells with parallel lateral sides into more trapezoidal forms, like voussoirs in an arch. This allows the cells to invaginate and form a concavity that ultimately pinches off to form the otocyst.
“We knew that morphogenesis was one of the first events after inner ear induction, which is controlled by a pair of FGF molecules, so we were curious as to whether FGFs played a role in this as well,” says Ladher. Sai and Ladher first confirmed that FGF was required for the morphogenetic stage of inner ear development by removing ectoderm that had been induced to become inner ear from embryos at different developmental timepoints. They found that even after induction, the tissue needed to remain in contact with its normal surroundings in order for morphogenesis to occur, suggesting that one or more external signals is required. Using microscopic beads soaked in the FGF factors known to induce the inner ear, they determined that FGF signaling to the basal aspect of the prospective inner ear was necessary to its subsequent morphogenesis. Knowing that changes in cell shape are often the result of cytoskeletal rearrangement, the team next looked at microtubules and actin during the morphogenetic phase, and note that while microtubules appeared not to change, actin filaments gradually became depleted from the basal aspect of the ectoderm in a manner suggesting that a local change in actin polymerization (rather than its localization) was responsible. In looking to connect FGF signaling with the change in actin dynamics, they homed in on one of two known FGF signaling pathways, involving the phosphorylation of phospholipase Cγ (PLCγ), which they had linked to basal FGF signaling through inhibition of PLCγ and another downstream factor, protein kinase C (PKC). Suspecting that the mechanism responsible for the basal expansion of ectodermal cells was actin depolymerization, they first surveyed for well-known actin depolymerization factors, but were unable to detect them in the basal otic ectoderm. They turned next to myosin II, a factor that typically mediates the contraction of actin filaments, but which had recently been shown by other labs to play a part in actin clearance and turnover as well. Activation of non-muscle myosin II relies on the phosphorylation of a regulatory region known as the myosin light chain (MLC), and when the team used an antibody specific to phosphorylated light chain, they found it localized to the basal side of the otic tissue. Inhibition of myosin II activity also prevented invagination, suggesting that its activity is needed for morphogenesis of the inner ear. With strong evidence that the FGF–PLCγ cascade and activated myosin light chain were both involved in the basal expansion of otic ectoderm during its morphogenesis, they sought to link them by studying the effects of inhibition of upstream factors on MLC phosphorylation, and found that it was blocked by the inhibition of either FGF or PLCγ. Using a general inhibitor of protein synthesis, they further showed that this activation of myosin II was a direct effect of FGF signaling, as even in the absence of translation, the activation of the FGF-PLCγ-myosin II pathway caused the depolymerization of F-actin in the basal aspect of otic ectodermal cells, necessary for the apical constriction and basal expansion that gives them their wedge-shaped morphology. “This is the first time to our knowledge that the shaping of cells involves something more than cell-intrinsic mechanisms,” says Ladher. “And as we see very similar processes in other aspects of development, like the formation of the neural tube from an originally flat plate of cells, it will be interesting to see how general the use of extrinsic signaling is in other such morphogenetic events.”
|
|||||
|
|||||
|
|
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |