| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
May 28, 2012 –Topology plays a central role in development. Sheets of cells held together by adhesion molecules fold or curl into functional barriers separating regions of the body or tubes that serve as tightly sealed channels. The neural tube is one such structure, which forms from an epithelial sheet called the neural plate that bends in, rolls up and pinches off to form an enclosed cylinder that subsequently serves as the source of an extremely wide range of cells types; indeed, this transient structure is sometimes referred to as the “fourth germ layer.” The early steps of neural tube formation involve a process of cell rearrangement known as convergent extension, in which sheets of cells spread toward each other and merge by intercalation, as well as a signaling pathway known as planar cell polarity (PCP) that reorients the alignment of intracellular components in the merging cells. How PCP and convergent extension work together to form the neural tube, however, is unknown. Using embryonic chick as a model, Tamako Nishimura and others in the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi, Group Director) have shown that the PCP signaling molecule Celsr1 is concentrated in the apical junctions of polarized cells in the merging neural plates. Published in Cell, this work shows how this single molecule coordinates both apical constriction and convergence of neuroepithelial sheets in the nascent neural tube.
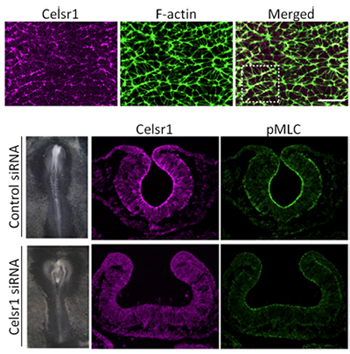
The inward bending of the neural plate relies on the purse string-like constriction of the apical surfaces of certain key cells in the region to generate a “hinge” effect, a process that is known to involve the activation of actomyosin in the region by the Rho kinase, ROCK. The resulting depression resembles a furrow, rather than a bowl, suggesting that this apical constriction is oriented along the anterior-posterior (A-P) axis of the neural plate. To gain a better understanding of how this occurs, Nishimura watched adherens junctions in the neural plate in chick embryos as the component cells remodeled the tissue by apical constriction. She found that activated actomyosin (as identified by the molecule pMLC) accumulated at junctions perpendicular to the A-P axis, and that cells tended to contract uniformly in the direction of the midline. Live imaging analyses of the region further revealed changes in both the cell morphology of the apically constricted cells and rearrangements of spatial relationships between cells along this axis, pointing to the possibility that this oriented constriction is a driver of convergent extension. This next raised the question of how this orientation is generated. Previous work from the Takeichi lab identified the Drosophila protein Flamingo as a member of the cadherin molecular superfamily involved in the regulation of planar cell polarity. Interestingly, mice lacking Celsr1, a vertebrate homolog of Flamingo, exhibit defects in neural tube closure – a common form of congenital defect in humans. The group speculated that Celsr1 might play a role in determining cell polarity and thus the orientation of apical constriction. Similar to pMLC, Celsr1 localized in junctions perpendicular to the neural plate A-P axis, and knockdown of the gene function by siRNA produced neural tube defects reminiscent of those seen in Celsr1 knockout mice, and while pMLC activity was maintained its localization to the cell-cell junctions was reduced. Cellular rearrangements underlying convergent extension were also diminished, strongly suggesting that Celsr1 plays a critical role in orienting apical constriction in this context. “Studies in Drosophila have highlighted the potential for local constriction of cell adhesion sites to exert significant effects on tissue morphology,” says Takeichi. “In this study, we have shown how this can occur in vertebrates as well. We next hope to focus on what drives the localization of Celsr1 and, more generally, whether this system is conserved in other tissues, and other taxa.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |