| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Jul 2, 2013 – Developmental biology is one of the most visually stunning fields of in the life sciences, and the ability to ‘see’ within the embryo is a fundamentally important approach to its study. The advent of fluorescent marker proteins, such as GFP, has made it possible to visualize the localization of the expression of labeled proteins, but the scattering of fluorescent signals in opaque biological samples means that such imaging can only peer to within a few hundreds of micrometers of the surface of the tissue, even when using advanced technologies such as two-photon microscopy. This creates significant problems for the study of complex three-dimensional tissues, such as neuronal circuits; the traditional approach of slicing the tissue to obtain two-dimensional data to be used in attempts to reconstruct 3D interconnected structures presents herculean difficulties. A number of alternative strategies involving the ‘clearing’ of tissue with solvents to render it more or less transparent have been developed, but these have also been complicated, time-consuming, and may affect fluorescent signals from labeled proteins. Meng-Tsen Ke and colleagues in the Lab for Sensory Circuit formation (Takeshi Imai, Team Leader) now look to improve the toolkit with a report of a new technique for optically clearing embryonic tissues, which they have named SeeDB. This new method, published in Nature Neuroscience, uses a water-based agent that preserves fluorescence and tissue structure, and enables the deep-layer visualization of neuronal circuits.
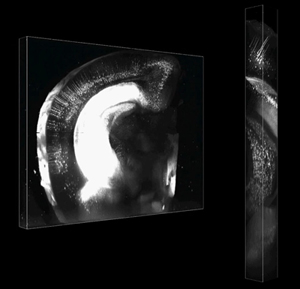
In seeking a better optical clearing agent, Ke and colleagues resolved to identify aqueous solutions with refractive properties similar to those of the tissues they wished to study. Other groups had reported some success using sucrose solutions, but the Imai team decided to test fructose as an alternative. After long experimentation, they refined the recipe to what they named SeeDB (for See Deep Brain), a solution that made it possible to render an embryo optically transparent in just three days, a major advance over previous techniques requiring 2–3 weeks. SeeDB also excels in preserving the size and morphology of embryonic structures, and avoids the quenching of fluorescence from molecular labels such as GFP. Additional information for scientists interested in this new optical clearing solution are available at SeeDB Resources. Ke et al. next sought to determine the tissue depths at which SeeDB imaging could be used, using samples from adult mouse brain. Working with a microscope manufacturer, they customized a two-photon microscope with a longer working distance lens, to overcome depth limitations of the commercially available version. Fluorescence imaging of 6 mm-thick samples cleared by SeeDB yielded beautiful, high-resolution images of even the deepest layers, demonstrating the utility of this technique at a scale suitable for whole-brain studies in mouse.
In a further test of the new clearing agent’s utility, the team labeled axons of the corpus callosum, which link the left and right hemispheres of the brain, and traced their projections in a mouse brain made transparent with SeeDB. They were able to trace individual axon fibers over long distances from one side of the brain to counterparts projecting from the opposite hemisphere, and found that such callosal axon projection relies not only on positional guidance from target tissue, but also sorting at the pre-target site. The team last moved to the lab’s core interest of studying the circuits formed in the olfactory bulb of the brain. In the mouse, olfactory sensory neurons of like type converge in glomeruli within the olfactory bulb, which is the site of odor information processing. Odor inputs to a glomerulus are then relayed to mitral cells through dendritic connections, but the wiring of these mitral connections has remained unresolved. Using SeeDB, Ke et al. were able for the first time to get an unobstructed view of the circuit layout for the full set of mitral cells associated with a single glomerulus, revealing an unexpected architectural complexity. “I am hopeful that this new technology will help usher in a new era of ‘3D biology’ aimed at a better understanding of neural circuit formation and other complex developmental processes,” says Imai. “However, we still need to fine-tune the microscopy and image data processing technqiues to make this even more suitable for a wide range of studies.” |
|||||||
|
|||||||
|
|||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |