| RIKEN Center for
Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
| Innermost secrets of inner ear induction | ||
March 1, 2005 – The ear’s architecture is ornate, with the three distinct components of outer, middle and inner ear coordinating to enable the senses of hearing, motion and balance. These otic subunits arise via separate genetic programs, the foundations of which are laid down very early in the embryo’s development. The inner ear, which grows to become home to populations of signal-transducing hair cells used in audition and maintaining equilibrium, is a marvelously intricate and multifunctional structure. It derives from a small patch of embryonic tissue known as the otic placode, a thickened disc that appears in an ectodermal region that would otherwise be destined to become skin.
The study of ear development reveals great diversity in molecular agents across taxa, although most of that variety is confined to the FGF family of secreted proteins and receptors. In the chicken embryo, signaling by FGF19 from the cranial mesoderm induces the expression of WNT8c and FGF3 in the neural ectoderm, which then cooperatively induce the otic placode to form in a nearby non-neural ectodermal region. The mouse achieves the same ends by different means, using mesodermal FGF10 and FGF3 in the hindbrain to instruct the placode to form.Zebrafish embryos, meanwhile, take a third route, utilizing FGF3 and FGF8 to engage and regulate otic induction. This last example sparked Ladher’s interest, as there had been no reports of Fgf8 expression in the parotic regions of either chicken or mouse, and, with his colleagues in Utah, he began to investigate the possible involvement of FGF8 in the induction of the ear in these species. Tissue ablation studies in chick showed that endoderm makes a contribution to the initiation of otic development, which subsequent investigations indicated was due to its induction mesodermal Fgf19. Looking at in the subjacent endoderm at developmental stages corresponding to the start of the Fgf19 expression, Ladher found that Fgf8 is indeed expressed in patterns suggesting a role in otic induction. The team found that exogenous FGF8 was sufficient to induce mesodermal Fgf19, then demonstrated its necessity by inhibiting Fgf8 expression in vivo using RNAi, which resulted in the downregulation of Fgf19 and failure of otic placode development.
|
||
 |
||
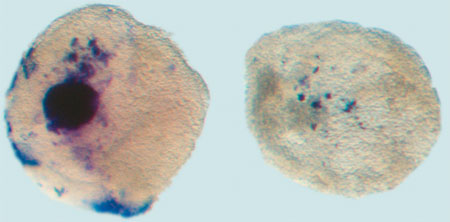
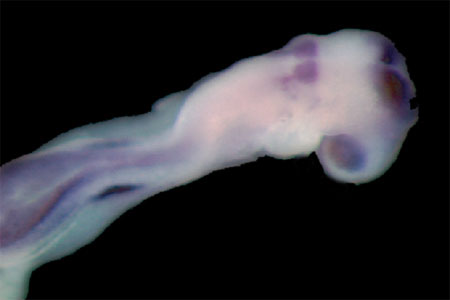
Wildtype embryo stained for Pax2 and Hoxb1, markers of ear development |
||
“What’s interesting about these results is that they show the involvement of signals from all three germ layers in inducing the ear,” says Ladher. “In both chick and mouse, endodermal FGF8 seems to be working as a molecular cue ball, setting off different combinations of serial and parallel interactions in the overlying mesoderm and ectoderm that ultimately result in the specification of the otic placode.” The study appears in the March issue of Genes and Development. |
||
|
||
[ Contact ] Douglas Sipp : sipp@cdb.riken.jp TEL : +81-78-306-3043 RIKEN CDB, Office for Science Communications and International Affairs |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |