| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
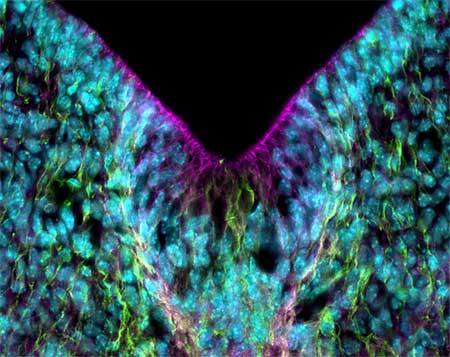
αN-catenin is found expressed throughout the nervous system, and previous studies of αN-catenin knockouts have revealed defects in the migration of Purkinje cells in the cerebellum, and, in studies by Hideru Togashi and Kentaro Abe, also in Takeichi group, impaired morphogenesis of dendritic spines and unstable synaptic junctions in hippocampal neurons. This latest work by Uemura adds a new level of depth to the insight into αN-catenin’s critical neurodevelopmental role. Slices of neonatal brain obtained from these mutants showed no global defects on initial inspection, but a closer analysis showed a number of subtler local effects, including a loss of curvature and reduction of the ventricular wall in the striatum, resulting in abnormal enlargement of the ventricular cavity. These regions showed no sign of either abnormal cell proliferation or apoptotic cell death, indicating that αN-catenin’s function in the ventricle is direct. Defects in brain morphology appeared in other regions as well, including thinning of the fornix and mammillothalamic tracts, a diminished hypoglossal nucleus, and disorganization of the inferior olive. Higher resolution analysis of the paths traced by migrating axons turned up defects in the midline crossing as well. In the αN-catenin knockouts, axons extending from the anterior olfactory nucleus migrated normally along their routes for a distance but became disoriented en route, and failed to cross the midline of the brain as they normally would. This set of defects strongly indicates the central role of αN-catenin in many different areas of the developing brain, but the molecular details of its function in these contexts remains to be studied. The affected regions in the Uemura study tended to be characterized by particularly strong αN-catenin expression, but there are numerous other spots in the brain where the gene is expressed but which are unaffected in the mutant. “We’re trying to account for the lack of a phenotype in other parts of the brain known to express αN-catenin; compensation by αE-catenin seems unlikely, as it is expressed mainly in areas where αN is not,” says Uemura. “αT-catenin, however, remains intriguing, as comparatively little is known about its function, and it may turn out to be redundant in function here. We’ve also seen a number of behavioral defects in the αN-catenin mutants, so it will be interesting to work out how those relate back to brain morphology.” |
|||||||||
|
|||||||||
 |
|||||||||
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |