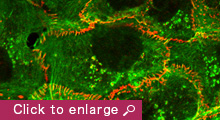
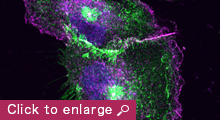
Animal cells organize into tissues with complex architecture. Our lab is exploring the molecular mechanisms by which individual cells assemble into a tissue-specific multicellular structure, such as epithelial sheets and neural networks. We are also interested in the molecular basis of how tissue architecture is disrupted during carcinogenesis, a process that is thought to accelerate the metastasis of cancer cells. For these studies, we are focusing on the roles played by cell-cell adhesion and recognition molecules, the cadherin family of adhesion molecules in particular, as these are known to be indispensable for tissue construction. Our current studies are divided into three categories:
1) Cell-cell adhesion is a dynamic process, and this nature of cell-cell adhesion is implicated in various cell behaviors, such as contact-dependent regulation of cell movement and cancer metastasis. A growing body of evidence suggests that cadherins cooperate with cytoskeletal and/or motility machineries, such as actin regulators, non-muscle myosins, and Rho GTPases, in modulating cell assembly. We are therefore studying the molecular mechanisms underlying the crosstalk between cadherins and such cytoskeletal systems, and their roles in epithelial junction formation.
2) A second area of interest to our lab is to gain a better understanding of how the cell-cell adhesion machinery contributes to animal morphogenesis. Using mouse embryos, we are analyzing the roles of cadherins and associated proteins in various morphogenetic processes, including neural crest migration. We are also investigating the roles of members of the cadherin superfamily known as protocadherins, deficiencies of which have been implicated in human brain disorders. Through these studies, we expect to gain deeper mechanistic insights into the ways by which cells build the elaborate structures of the animal body.
3) In addition, we have been analyzing the functions of microtubule minus end-associated proteins, Nezha/CAMSAPs. These proteins regulate microtubule assembly patterns, centrosomal function, and organelle positioning. We are exploring the roles of these molecules in cellular morphogenesis, such as polarized epithelial formation and axon growth, with the aim of uncovering novel functions of non-centrosomal microtubules.

A:…
A:…
takeichi[at]cdb.riken.jp