
News and Announcements from the CDB
The neural network in the brain is orderly yet extremely complex, and improper neural wiring during development can lead to brain disorders in humans. While many brain disorders are linked to genetic causes, in most cases, the underlying pathogenic mechanisms remain unclear. One disorder called PCDH19 Epilepsy, is known to be caused by mutations in a gene located on the X chromosome called Protocadherin-19 (Pcdh19). Normally, X-chromosome-linked diseases, such as color blindness and hemophilia, show severe phenotypes in males, which carry only one X chromosome. This disorder displays a unique pattern of inheritance, with symptoms appearing in females. Why phenotypes of PCDH19 mutations are female-specific, and the pathophysiological mechanisms of PCDH19 mutations remained largely unknown.
Now in a study led by research scientist Shuichi Hayashi of the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi, Team Leader), who is now at the University of Oxford, he and his collaborators carried out a phenotypic analysis of mice in which the X-linked gene, Protocadherin-19 (Pcdh19) was knocked out. Their analyses revealed while brain tissue organization and neural morphology showed no significant abnormalities, the Pcdh19-knockout (KO) mice exhibited abnormal behavior in response to certain stress conditions and differential behavior between males and females. Overexpression of Pcdh19 in the cerebral cortex of wildtype mice also revealed that Pcdh19 expressing cells tended to cluster together, and this clustering appears to cause disease phenotypes when females have one chromosome carrying this mutation. Their findings were published in the online journal Scientific Reports.
Protocadherins are a group of cadherin superfamily proteins that are found in cell membranes, regulate homophilic cell-cell interactions and are expressed in central and peripheral nervous systems during development. In a previous study also led by Hayashi, they showed that Pcdh17 plays an essential role in bundling axons to mediate collective axon extension of amygdala neurons during development (See Science News: Dec. 18, 2014). As PCDH17 and PCDH19 are part of the same subfamily, both are thought to be involved in brain development or its function. PCDH19, in particular, has been implicated as a causal factor in a range of brain disorders when mutated.
Hayashi and his collaborators first examined the role of PCDH19 in the brain by generating Pcdh19-KO mice. In normal mice, Pcdh19 is expressed widely throughout the brain, but is particularly strong in the cortical layer V of the cerebral cortex and the hippocampal CA1 region. It was found distributed along the dendrites of neurons, and even in some of the dendritic spines. When brains of Pcdh19-KO mice were examined, they found no significant abnormalities in the cortical and hippocampal structure, nor in dendritic morphology.
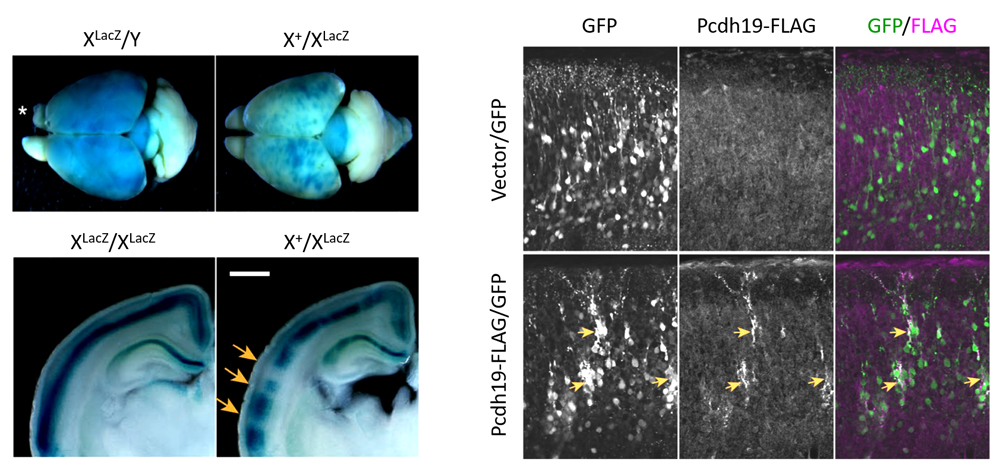
The team next looked at genotypic differences in the distribution of Pcdh19 in the brain. They discovered that heterozygous females (X+X–) showed patchy distributions of Pcdh19 in the brain, with Pcdh19-positive and Pcdh19-negative cells forming respective clusters, whereas homozygous females (X–X–) uniformly lost Pcdh19 expression across the brain. In females, only one X-chromosome is functional as the other one is inactivated through random X-inactivation. This random X-inactivation of an X-chromosome lacking the Pcdh19 gene is thought to be to reason for the patchy distribution of Pcdh-positive and Pcdh-negative neurons, and is consistent with reports from past studies. They also examined whether Pcdh19’s homophilic properties also contribute to the patchy distribution by exogenously overexpressing Pcdh19 in a subset of cells in wildtype brains using in utero electroporation and found that clustering was enhanced in neurons strongly expressing Pcdh19. These results suggest that differences in Pcdh19 expression that arise from mosaic distribution promotes clustering of some neurons, disrupting normal neuronal patterning.
Wondering what effects loss of Pcdh19 might have on behavior, they carried out a series of behavioral analyses in a collaboration with Fujita Health University. While no significant differences were observed in social behavior, learning or memory, both male (X–Y) and female (X+X–) carriers of Pcdh19 mutations displayed increased activity during the tail suspension tests, which suggested that when placed under stress, they are predisposed to becoming hyperactive. In addition, only the female carriers (X+X–) showed reduced fear responses in comparison to wildtype. These findings suggest that female-specific abnormalities observed in human PCDH19 Epilepsy was in part replicated in the mouse model, and as evidenced by male carriers also displaying some behavioral abnormalities, Pcdh19 is likely important in regulating mouse behavior.
“As loss of PCDH19 does not significantly affect brain tissue or neuronal morphology, it is likely small changes in neural connections result in behavioral abnormalities. Overexpression experiments showed that Pcdh19-expressing cells are prone to cluster together, which is consistent with the hypothesis that the female-specific appearance of PCDH19 Epilepsy is due to mosaic distribution of Pcdh19-positive cells that triggers excessive aggregation and thereby the disease phenotype,” says Takeichi. “Human brain pathophysiology is difficult to replicate in mice, but provided we continue to deepen our understanding of the disease at molecular and cellular levels, our efforts may lead to future applications in medicine.”
| Link to article | |
|---|---|
| Related link |