| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
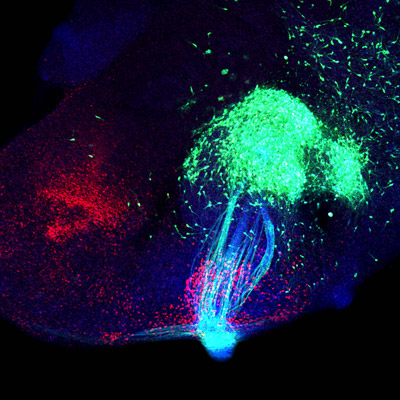
During the development of the mouse brain, axons from various brain regions enter the forebrain region known ventral telencephalon where they then must decide where to turn next. This decision-making process is informed by guidance cues, molecular signposts that steer the migrating axons on their way. It is also a site of abundant OL-pc expression, which led the Uemura and Hirano to test the function of the molecule within that context. To do so, they engineered a line of mice in which this gene was disabled and made a close examination of the wiring of its brain at relevant developmental stages. The homozygous mutants (which were viable through embryogenesis, but died within a few weeks of birth) exhibited a range of neuronal circuit defects, the most pronounced of which involved the cortex and thalamus. Axons in these regions showed a range of targeting problems, although neuronal differentiation itself appeared unaffected. The disappearance of barrel structures in the region suggested that the majority of thalamocortical axons had not reached their normal destinations in the cortex. Looking within the ventral telencephalon, Uemura and Hirano found no abnormalities in a range of transcription factors involved in patterning, specification and axon guidance, but did note that Nkx2.1, a marker of the brain region known as the globus pallidus, was absent from the tail end of this region. This was telling as it has been speculated that the globus pallidus is important for the pathfinding of thalamocortical axons. Using a cell labeling strategy, the group determined that putative guidepost cells in this region were either absent or mislocalized in the homozygous mutant. When they next studied a second region, the striatum, they found that axons emanating from the mutant striatum became entangled and failed to project to the globus pallidus normally. Seeking to explore this more closely in vitro, they isolated striatal tissue from transgenic mice expressing GFP and grafted them into slice from the ventral telencephalon. Interestingly, the graft projected axons to the Nkx2.1+ region and immediately formed an axon tangle in wildtype, but failed to extend axons in the OL-pc mutant, suggesting that this protein is essential for sustaining the migration of striatal axons toward the globus pallidus. “In this work, we were able to demonstrate a previously unknown function for OL-protocadherin in the formation of multiple neural pathways in the ventral telencephalic network,” says Hirano. “And, given our findings, we now also believe that striatal axons may play an important role in patterning of ventral telencephalon and subsequent neural projections in this region, which we’ll be interested in learning more about.”
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |