
News and Announcements from the CDB
Carcinomas are the most common types of cancer, which are formed by epithelial cells that undertake major functions specific to various organs. Normal epithelial cells possess an apical-basal polarity, and are arranged in a sheet-like structure by forming a stable connection with neighboring cells via the apical junctional complex (AJC). When epithelial cells turn cancerous and undergo tumor progression, they are prone to exhibit loss of cell polarity and defects in cell-cell adhesion, which are thought to contribute to invasiveness and metastasis, at which stage effective cancer treatment options are limited. Targeting the restoration of cell-cell adhesion may be one way to limit or prevent tumor invasion or metastasis, however, to date no such therapy has been developed.
In a study by led by research scientist Shoko Ito of the CDB’s Laboratory for Cell Adhesion and Tissue Patterning (Takeichi Masatoshi, Team Leader), she and her collaborators demonstrated that microtubule polymerizing inhibitors (MTIs) can restore cell-cell adhesion in adhesion-defective colon carcinoma cells. The further analyzed the mechanism at work, found that inhibiting microtubule polymerization activated RhoA to mediate the contractions of the actomyosin complex near the cell’s apical cortex. The mechanical force of this contraction is transmitted to the cell boundaries, to recruit a mechanosensitive factor vinculin and other adhesion regulators to induce rebuilding of AJCs. Their findings were published in the online journal, Nature Communications.
Cell-cell adhesion in epithelial cells are generally stable, and are mediated by cadherins and associated cytoplasmic proteins that organize into the cadherin-catenin complex (CCC). In many types of cancer cells, however, this adhesion complex becomes defective, leading to disruption of cell-cell adhesion and thereby promoting invasion and metastasis. The laboratory and other groups have previously identified colon carcinoma cell lines that could not organize proper junctions, despite the cells expressing core components of the CCC. They also discovered that these carcinoma cell lines could at times reorganize relatively normal junctions following treatment with different chemicals, but the mechanism involved in the reorganization was not known.
In collaboration with RIKEN’s Program for Drug Discovery and Medical Technology Platforms, Ito used HT29 cells, a colon carcinoma cell line that is known to express CCC components yet exhibit adhesion defects, to carry out a comprehensive screening for chemical compounds that restore AJCs. The screening picked up 124 different types of chemical compounds out of a library of 160,960, which appeared to effectively restore junctions. Approximately 80% of these 124 compounds identified were microtubule-depolymerizing drugs, or MTIs. The team also found that following MTI treatment, the HT29 cells not only reorganized relatively normal AJCs, but also re-established apical-basal polarity.
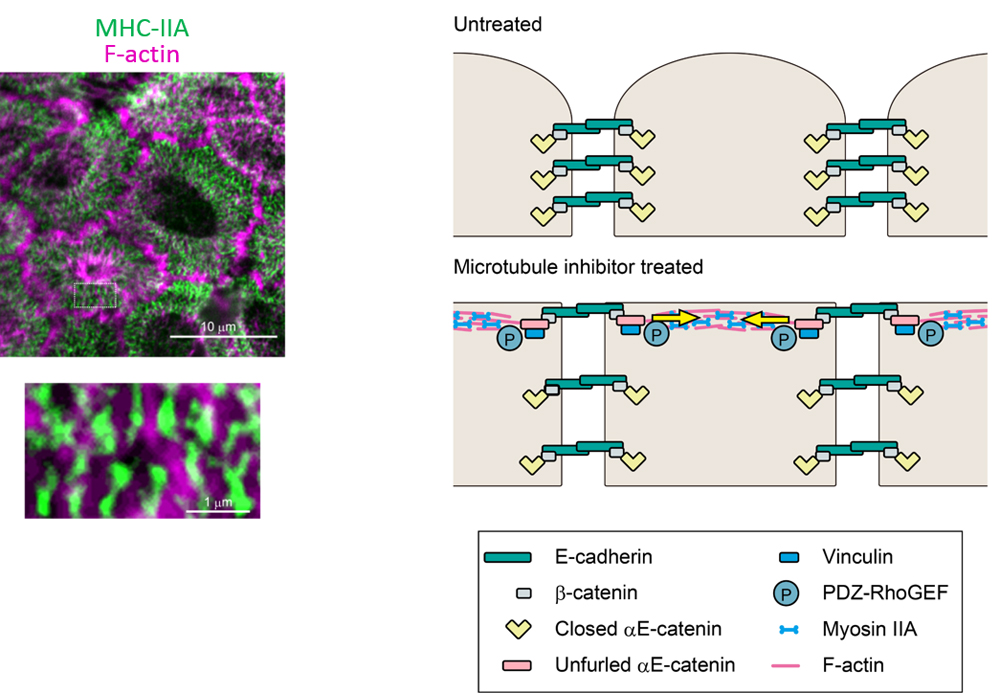
Ito and her collaborators next examined how MTIs restored AJCs. When microtubules undergo depolymerization, the Rho guanine nucleotide exchange factor, GEF-H1, becomes activated. GEF-H1 in turn activates RhoA, and RhoA will activate cortical myosin-II via the Rho/ROCK pathway. The team analyzed time-lapse movies of HT29 cells treated with the MTI nocodazole and observed actomyosin condensing and contracting inwards. Under super-resolution microscopy, they observed that the myosin-IIA heavy chain (MHC-IIA) was organized into a striated pattern, similar to that seen in muscle sarcomeres. F-actin, which can bind to myosin-II, were also seen to reorganize into filamentous structures along the cell boundaries creating tensile forces along the cell’s apical surface. The tensile force in turn helps to recruit vinculin, a mechanosensitive molecule, to the cell boundaries to further recruit other actin regulatory molecules to restore AJC.
“In this study, we showed how activation of RhoA by MTIs can restore cell adhesion in colon carcinoma cells, and further, that the MTI treatment step could be skipped as long as RhoA was activated in some manner. As other groups have reported that human cancer cells exhibit abnormal apical RhoA activity, disruption of RhoA-mediated cell adhesion may be a feature common to many cancer cells,” says Takeichi. “Why cell adhesion becomes disrupted in carcinoma cells, and the mechanism underlying this phenomenon remains unknown. But, if we can find a way to regulate RhoA activity to restore functional junctions, it may lead to a new therapeutic approach for controlling cancer metastasis or invasion.”
| Link to article |
Induced cortical tension restores functional junctions in adhesion-defective carcinoma cells. |
|---|