| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Oct 1, 2013 –Microtubules are components of the cytoskeleton known for their roles in maintaining cellular structure and cytoplasmic transport. These elongated proteins form two distinct populations, one anchored to the centrosome by its minus-end and a second that grows from other sites within the cell, through interactions with other molecules. It has previously been shown that the cytoplasmic protein CAMSAP3 (also known as Nezha) stabilizes non-centrosomal microtubules, but it remains unclear whether the roles of non-centrosomal in cellular architecture and other functions differ from those of the centrosomal type. Now, a study by Shigenori Nagae of the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi, Group Director) and the Kyoto University Graduate School of Biostudies, working in collaboration with the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences (Beijing, China), reveals that non-centrosomal microtubules mediated by CAMSAP3 regulate the organization of a second cytoskeletal protein, F-actin, which plays roles in cellular morphology and behavior. Published in Genes to Cells, these findings shed new light on the molecular machinery underlying microtubule function.
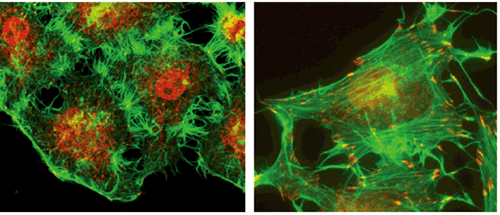
CAMSAP, which serves as the nucleus of non-centrosomal microtubule formation, was recently identified by the Takeichi group, who showed that CAMSAP stabilizes microtubules through binding their minus-ends. In this latest study, Nagae et al. used the HeLa line of immortalized human cells to study the function of microtubules bound to CAMSAP. The group focused specifically on CAMSAP3, which they found to be expressed in a punctate fashion throughout the cells, binding with microtubule minus ends, with the plus ends growing outward from these CAMSAP loci. Using RNAi to inhibit CAMSAP expression, the group found that the ordinarily smooth-edged cells became spiky and distorted, taking on a distinct ‘thorny’ morphology, with a concomitant increase in the number of centrosomal microtubules. Curious about the cause of the morphological changes, they next examined the activity of a second cytoskeletal protein, actin, in CAMSAP-inhibited cells, and found that actin stress fiber was upregulated on loss of CAMSAP function. But how then do changes in non-centrosomal microtubule formation affect actin fibers? In looking for the underlying mechanism, they next focused on RhoA, a protein known to induce actin stress fiber formation. Intriguingly, they found that depletion of CAMSAP3 led to an increase in detyrosinated microtubules (which were found to be at higher levels in centrosomal microtubules than in non-centrosomal microtubules). Due to this, the Rho activator GEF-H1 became unbound from microtubules, leaving it free to activate RhoA, resulting in the promotion of actin stress fiber formation and, ultimately, changes in cell shape. Thus, it appears that under normal conditions binding of microtubules by CAMSAP3 inhibits RhoA activity maintaining actin fiber formation at a fixed level. These new insights help to clarify the mechanistic and functional differences between centrosomal and non-centrosomal microtubules. “We are now finding that a balance between these two types of microtubules serves to regulate actin fibers, which may contribute to cell type-dependent morphological processes,” says Takeichi. “By clearly distinguishing between these different microtubule populations, we hope to contribute even more to our understanding of how these molecules work in the cell.” |
|||||
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |