| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Dec 3, 2012 –Microtubules organize themselves differently in different types of dividing cells. While they often grow radially out from the centrosome during interphase in many cell types, this is not the case in epithelial cells, in which their minus-ends are mainly anchored in the cytoplasm instead. Despite its importance to mitotic dynamics, however, the means by which the distribution of centrosomal and noncentrosomal microtubules is established and maintained in different cells has a remained a mystery. A study by Nobutoshi Tanaka and others in the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi, Group Director) has now shown that a pair of molecules, CAMSAP3 (also known as Nezha) and CAMSAP2, work together to regulate and maintain noncentrosomal microtubule organization in cultured human cells. Published in the Proceedings of the National Academy of Sciences, this work reveals the mechanism by which interphase epithelial cells establish this distinct form of cytoskeletal organization.
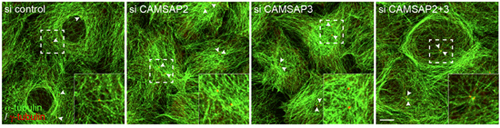
Microtubules are polarized molecular chains, in which the terminal site of growth by polymerization is called the plus end; the minus end is found at the other pole. Minus ends have been shown to anchor in the centrosome, from which microtubules extend radially, a process mediated by numerous molecular factors. But some microtubules stabilize without tethering to the centrosome, by mechanisms that remain poorly understood. Seeking to gain more insight into this these noncentrosomal microtubules, Tanaka focused on CAMSAP3, which the Takeichi group had previously shown to bind to microtubule minus ends, and a related molecule CAMSAP2. The group first looked at the localization of the two molecules and found that both tended to clump together in small clusters throughout the intracellular space. When they simultaneously visualized the plus ends of microtubules by immunostaining, they noted that the CAMSAP clusters tended to be located at the opposite poles, suggesting an affinity for minus-ends. Having established a physical connection, Tanaka et al. next sought after the functional role. The plus-end protein, EB-1, which they used in their immunostaining experiments, is known to bind to microtubule plus ends and contribute to their polymerization. Using specific siRNAs to knock down the function of each of the CAMSAPs, the group found that loss of the function of either molecule resulted in a reduction of EB1 radiating growth. This effect was amplified when both CAMSAPs were knocked down simultaneously, suggesting that these proteins promote stable extension of microtubules. In wildtype epithelial cells in low-density cultures, most microtubules are arrayed in a pattern surrounding the nucleus. The centrosomes locate at random sites, and only rarely nucleate radial microtubules. But the loss of function of either CAMSAP3 or -2 triggered a rearrangement in which the microtubules densely covered the nucleus. Even more intriguingly, the double knockdown of both CAMSAPs caused some epithelial cells to develop uncharacteristic centrosomal radiation of microtubules. Cells lacking either CAMSAP also showed post-translational changes in the tubulin protein, which may account for the altered microtubule dynamics. The arrangement of microtubules growing from CAMSAP sites appears to have functional consequences as well. In CAMSAP-depleted cells, early endosomes accumulated around the centrosome, while in control cells these organelles are dispersed throughout the cytoplasm. The Golgi apparatus, which is normally distributed in the perinuclear region, was more widely distributed in CAPSAM-knockdown cells. “While this series of experiments points to some very interesting possibilities, we will need to study these phenomena more closely in epithelial tissue in vivo rather than low-density cell culture,” notes Takeichi. “As epithelial cells show distinct microtubule orientation toward their apical surfaces, we may be able to detect whether CAMSAPs play regulatory role in the establishment of this polarity, and how their depletion affects cell morphology and behavior in intact tissue.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |