| Cadherins go with the flow |
 |
 |

December 25, 2006 – Adhesion between cells must be robust enough to provide a stable basis for the organization of tissues, but dynamic enough to provide the flexibility and mobility required in certain situations, such as development and wound healing. This cellular freedom of movement is essential to both the formation and maintenance of multicellular life, but the means by which it is regulated by molecular adhesion machinery remains one of the mysteries of biology.
Now, in a study published in Nature Cell Biology, Yoshiko Kametani and Masatoshi Takeichi of the Laboratory for Cell Adhesion and Tissue Patterning describe a new aspect to the behavior of cadherin proteins, showing that these cell-cell adhesion molecules, in certain cellular contexts, move in coordinated, unidirectional flows. They further show how such cadherin flow is linked to cytoskeletal movements, opening up the possibility that plays a significant role in the remodeling of epithelial tissues.
|
| Cadherin flow at the cell-cell junction. Clusters of cadherins can be seen moving in a basal to apical direction up the sloping junction zone. |
Cadherins are transmembrane molecules that extend long tails through the cell surface to form links with other cadherins of like type on adjacent cells. These proteins are anchored in the cellular interior by binding with members of the catenin family of proteins, which themselves are believed to associate with the actin cytoskeleton, a dynamic structural component of cells of fundamental importance to cell motility. Kametani used green fluorescent protein to label a specific type of cadherin known as VE-cadherin and monitor its behavior in epidermoid cancer cells in vitro. These epithelial cells tend to organize into sheets, but when she observed the junction zones between cells, she found that these often appeared to be titled, sloping upward from the cells’ base to their apical surface. And, interestingly, clusters of cadherin molecules appeared to form and gradually move upward on these junctional inclines, resulting in a clearly identifiable basal-to-apical flow.
Intrigued by “cadherin flow,” the group began to analyze the phenomenon in molecular-level detail, and found that the mobile cadherins maintain both their transdimer links with cadherins on adjacent cells, and their colocalization with associated catenin molecules. By introducing mutations into both cadherins and catenins, Kametani revealed that this flow relies on the connection between the adhesion machinery and filamentous actin mediated by catenin family members. Double immunostaining of VE-cadherin and F-actin showed that the cadherin clusters tended to associate with actin fibers and in some cases even jumped from fiber to fiber. Further analysis of actin dynamics near the cellular junction revealed that these cytoskeletal molecules as well were continuously reorganizing themselves and moving in a retrograde flow. Actin flow had been shown in previous studies to depend on the function of the motor protein myosin II, and when the group used a myosin inhibitor to block its function, they found that cadherin flow was stopped as well.
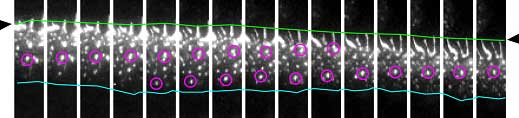 |
| Time-lapse images of cadherin movements. Cadherins flow in a unidirectional manner at the cell-cell junction. |
Cadherins are found in a wide variety of epithelial cell types, so to determine whether the flow effect was lineage-specific, Kametani next tested for flow-like movements of cadherin clusters in other cell lines transfected with VE-cadherin. She found that some, but not all cell types showed similar cadherin flow—in MDCK cells for example, cadherin movements seemed random. But even in these cells, flow could be induced by scraping the surface of the culture to simulate a wound; when cell migration began at the wound edges, cadherins began to flow in the direction of the migration as well. The picture that develops from this set of findings suggests that cadherin flow is an actin-dependent phenomenon that enables cells to move under or over cells in a continuous layer while retaining the ability to switch this function off once the ability to migrate is no longer needed.
This study shows a sophisticated balance between epithelial cellular adhesion and motility that underlies the ability of the multicellular body to organize and maintain itself. “It seems that cadherins may be working as a kind of clutch during cell migration that uses actin flow to provide the impetus,” says Kametani. “And as cellular adhesion and migration are important both to physiological development and pathological processes such as cancer invasion and metastasis, we hope that this work may one day have clinical implications as well.”
|


