| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
July 10, 2011 –Mitotic cell division involves a critical process in which replicated chromosomes separated by spindle fibers are pulled apart into separate daughter cells. In the roundworm C. elegans, such mitotic spindles, comprising microtubules and associated proteins, are assembled through both γ-tubulin-dependent and –independent processes. Previous studies have shown that the γ-tubulin-independent microtubule assembly relies on the function of AIR-1, the C. elegans homolog of Aurora A kinase, a key regulator of numerous mitotic events. But just how AIR-1 works in this aspect of microtubule assembly remains unclear.
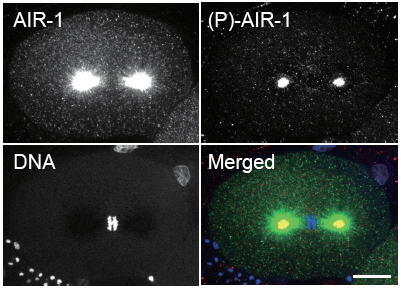
Mika Toya and others in the Laboratory for Developmental Genomics (Asako Sugimoto, Team Leader) have now deciphered AIR-1’s role. In a report published in Nature Cell Biology, the team used live cell imaging and RNAi approaches to show that AIR-1 stabilizes the spindle in a non-kinase-dependent fashion in addition to its kinase-dependent role at the centrosomes. Toya, who has since joined the CDB Laboratory for Cellular Adhesion and Tissue Patterning, began by observing microtubules in mitotic cells in which the expression of air-1 or tbg-1 (the C. elegans γ-tubulin gene) were knocked down by RNA interference in living embryos. They found that while microtubule numbers and lengths remained roughly constant in the air-1(RNAi) embryos, these increased significantly during chromosome condensation in the tbg-1(RNAi) cells, suggesting the two mechanisms were different. In other organisms, in addition to centrosomes (major microtubule organizing centers), condensed chromatin can trigger microtubule assembly. To determine whether this is the case in C. elegans as well, they tested air-1 and tbg-1 knockdown in embryos in which chromatin-stimulated microtubules were made readily visible, and found that while γ-tubulin appeared to play no role, loss of AIR-1 function caused microtubules to grow to shorter lengths and in fewer numbers. Immunofluorescence also showed that AIR-1 localized around condensed chromatin, but γ-tubulin was not seen in these regions. The kinase activity of AIR-1 depends on phosphorylation of the protein at a specific site. To monitor such activity, the team developed a phospho-specific AIR-1 antibody, allowing them to specifically visualize the subpopulation of AIR-1 proteins showing kinase activity. Interestingly, the phospho-AIR-1 signal was not detected in proximity to condensed chromatin, suggesting that its kinase activity was not involved in this aspect of its function.
The team tested this idea by generating transgenic embryos that expressed the kinase-inactive form of AIR-1. Toya et al. found that while AIR-1 association with microtubules was unaffected by the absence of kinase activity, its kinase-inactive form appears to be critical for stabilizing microtubules once assembled. Kinase activity was likewise found to be dispensable for the chromatin-stimulated microtubule assembly. The association of the kinase-inactive form of AIR-1 with microtubules was regulated by levels of the wildtype protein – in the presence of wildtype AIR-1, microtubule localization of the kinase-inactive construct was reduced. Given these findings, the team speculates that the balance between active and inactive forms of AIR-1 may be important in controlling mitotic spindle assembly and behaviors. “It will be important to investigate whether Aurora A in other organisms, including mammals, have similar kinase-independent functions,” says Sugimoto, now at Tohoku University. “We speculate that kinase-independent roles of protein kinases may be more widespread, although these have been missed in previous studies.” | |||||||||
 |
|||||||||
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |