| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
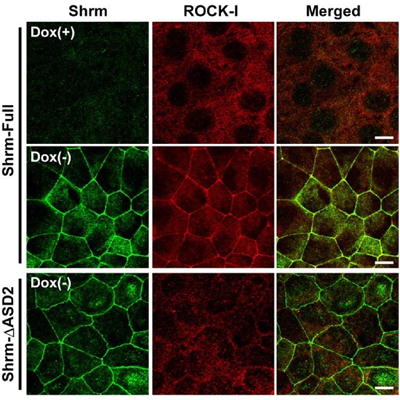
Now, a study conducted by Tamako Nishimura of the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi; Group Director) identifies the role of one player in neural tube morphogenesis, the actin-binding protein Shroom3. In a report published in Development, Nishimura showed that this protein binds certain Rho kinases, recruiting them to the apical cell junction, a site at which uppermost lateral surfaces of epithelial cells connect, and which serves as a hinge and anchor point for much tissue remodeling activity. Shroom3 was originally identified as the culprit in a mouse mutant whose phenotype was characterized by incomplete neural tube closure (the name deriving from the mushroom-like appearance of the mutant embryo’s head). Gene expression studies had shown that it is expressed strongly in the neural epithelium, but its mechanism of action remained a mystery. To address this question, Nishimura first searched for binding partners, and found that a number of Rho kinases (known as ROCKs) associated with Shroom3 in immunoprecipitation assays. In vitro tests further revealed that Shroom3 and ROCKs co-localized at the apical cell junction, an effect that could be blocked by interfering with their binding activity. Looking next in vivo, Nishimura studied the Shroom3-ROCK association in embryonic chicken, and confirmed that the two were concentrated in the apical junctions of neuroepithelial cells lining the neural tube. Blocking Shroom3 by RNAi resulted in the failure of ROCKs to localize to the apical junction, and consequent defects in neural tube closure. When she watched the interactions between cells in the closing neural tube, Nishimura observed assemblies of 5 or 6 cells into rosette patterns, suggesting coordinated contraction of the cell shapes, presumably dependent on actomyosin, which is known to be regulated by ROCKs. Such rosettes, again, were lost on ROCK inhibition. The picture that emerges from Nishimura’s findings suggests that Shroom3 recruits ROCKs to the apical junction, where they interact with myosin and actin cytoskeletal components to give shape to the forming neural tube. “There are still many unknowns in terms of the mechanisms behind changes in cell shape, such as those that occur in the neural tube,” says Nishimura. “I think the Shroom/ROCK complex is particularly interesting in this context, for the way it localizes in a polarized way to only one aspect of the cells and thus contributes to the sculpting of the entire tissue.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |