
News and Announcements from the CDB
Epithelial cells are found covering flat surfaces and lining tubular organs of the body. They exhibit an apicobasal polarity, a characteristic that is essential for carrying out their numerous functions such as secretion, absorption, and protection. One feature contributing to epithelial cell polarization is the distinct intracellular organization of microtubules, a key component of the cytoskeleton. In polarized epithelial cells, microtubules are arranged in longitudinal rows parallel to the apicobasal axis, appearing like a string curtain, but the mechanisms at work to properly orient the microtubules remained unclear.
Now in a new study led by research scientist Mika Toya in the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi, Team Leader), the team demonstrates, using mouse intestine epithelial cells and human colorectal adenocarcinoma (Caco-2) cells as models, that microtubule-binding protein CAMSAP3 (calmodulin-regulated–spectrin-associated protein 3) is important for microtubule orientation in polarized epithelial cells. They also show that microtubule orientation is critical for correctly positioning organelles within the cell. Their work was published in the Proceedings of the National Academy of Sciences USA.
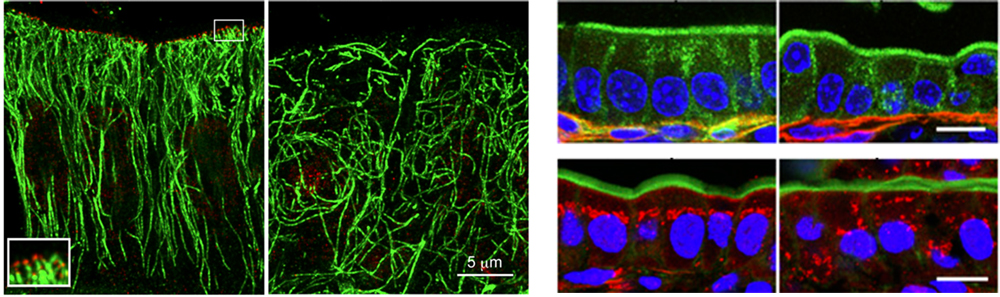
Left: Distribution of CAMSAP3 (red) and microtubules (green) in mouse intestinal epithelial cells. Upper right row: Position of nuclei (blue, nucleus; green, apical protein ezrin; red, basal membrane) Lower right row: Position of Golgi apparatus (blue, nucleus; green, apical cortex, red, Golgi). Scale bar for both rows, 10 μm. All panel sets, wildtype is on left and Camsap3 mutant is on right.
Microtubules are involved in many cellular functions such as cell structure maintenance, intracellular transport, cell motility and chromosome segregation. The tubular structure of the microtubule has polarity, a minus- and plus-end, with the plus-end being associated with rapid elongation. In epithelial cells, microtubules are aligned along the apicobasal axis, with minus-ends oriented near the apical side and plus-ends toward the basal side. Past studies from the same laboratory using cultured cells identified CAMSAP3 (also called Nezha) as a protein binding specifically to microtubule minus-ends (*Science news: Dec. 3, 2012; Oct. 1, 2013). In the current study, the team focused on revealing the mechanism underlying microtubule orientation in polarized epithelial cells.
CAMSAP3 is known to bind to microtubule minus-ends through its CKK-domain at the C terminus, so the team first generated a Camsap3 mutant mouse line, which expresses a CAMPSAP3 lacking the CKK-domain. Homozygous mutants displayed growth impairments, and about 15% of these mice died before postnatal day 30. When epithelial cells of the small intestine were examined, they noticed those from Camsap3 mutants lacked the normally punctate distribution of CAMSAP3 on the apical side. Comparisons of microtubule distribution in cells using stimulated-emission depletion (STED) super-resolution microscopy revealed that microtubules in cells from wildtype were bound to the apically distributed CAMSAP3 and aligned longitudinally along the apicobasal axis, whereas in mutant cells, microtubules were disorganized, displaying no specific orientation. Thus, these experiments suggested that apically distributed CAMSAP3 steers proper microtubule orientation by binding to the microtubule minus-ends, anchoring them in place.
They next examined arrangements of organelles in cells from Camsap3 mutant mice. In wildtype mice, cell nuclei were found in near the basal side at relatively similar distances from the basal membrane, whereas in Camsap3 mutants, nuclei positions were disorganized, with some even positioned close to the apical side. Position of the Golgi apparatus, generally found just above (apical to) the nucleus, was also abnormal in Camsap3 mutants, scattered to different areas of the cytoplasm. Cells from mutants also appeared to have problems maintaining consistent cell height along apicobasal axis. Electron microscopy revealed mitochondria elongation, and in some cells, the normally apically positioned adapter protein ezrin was mislocalized. Thus, it appears that organelles cannot be correctly localized when microtubules are misoriented as seen in Camsap3 mutant cells.
Toya also analyzed CAMSAP3 function in Caco-2 cells, a human colorectal adenocarcinoma cell line, culturing them in a media that allowed the cells to maintain epithelial-like polarity. When CAMSAP3 was depleted in Caco-2 cells, they found microtubule misalignment and mislocalization of organelles. Analyses of the CAMSAP3 domains revealed that the CC1 domain contained an amino acid sequence that was critical for CAMSAP3’s apical localization. CAMSAP3 also requires binding to microtubules through its microtubule-binding CKK domain for apical localization.
“Our combined experiments using STED super-resolution microscopy and generating Camsap3 gene knockouts has allowed us to analyze microtubule alignment in epithelial cells in greater detail than ever before,” explains Takeichi. “We hope to look at CAMSAP3 function in other cell types, and understand how microtubules bound to CAMSAP3 control intracellular structure. It will also be interesting to determine the underlying causes of the growth defects observed in Camsap3 mutant mice.”
| Link to article |
CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells. |
|---|---|
| Related link | |