| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
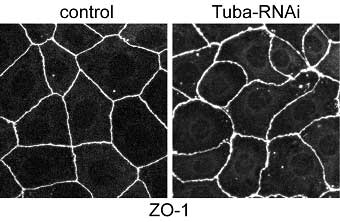
Now, in a report published in The Journal of Cell Biology, Tetsuhisa Otani and colleagues in the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi; Group Director) and Kyoto University identify Tuba as concentrated at the apical-most regions of epithelial cell junctions through its interaction with ZO-1, a protein associated with the tight junction area of cell-cell junctions. In the absence of Tuba, cell junctions in a human intestinal cell line became morphologically disrupted, and took on a curved and distorted appearance.
This is the first study to report on the apical-specific localization for Cdc42 and one of its GEFs, indicating that Tuba may play a unique, position-specific role in junctional organization. Otani and colleagues took their results to suggest that Tuba is required in regulating the configuration of apical junctions, and that it regulates actin polymerization—an important process in the development of the actin cytoskeleton through its production of stress fibers and filopodia—through Cdc42 and its effectors. The subsequent strands of colocalized F-actin and E-cadherin increase surface tension so that the surface area of cell junctions is minimized, and may be involved in stabilizing the linear morphology of cell junctions; this is all disrupted by the loss of Tuba. In examining ZO-1, Otani et al. found that knockdown of this particular protein revealed its function as both a partner of Tuba and a regulator of myosin activity, which affects cell junction morphology. As a reduction in Tuba had no corresponding effect on myosin distribution, the authors concluded that the slackened phenotype of Tuba-depleted cell junctions is brought about by cooperation between myosin-dependant contractility and Tuba-mediated actin organization, rather than by actomyosin activity. Using these findings to delve deeper into the sub-cellular layer, Otani and colleagues went on to reveal that Tuba inactivation modified the assembly pattern of junctional F-actin and E-cadherin, which act in holding neighboring cells together. They further found that while depletion of Cdc42 mimicked Tuba reduction, this factor’s overexpression rescued the Tuba RNAi phenotypes, indicating that Tuba functions via Cdc42 and that Cdc42 acts downstream of Tuba signaling. Depleting N-WASP, another protein and an effector of Cdc42, resulted in distorted apical junctions similar to Tuba depletion, suggesting that N-WASP is necessary for the normal accumulation of E-cadherin and F-actin at cell junctions. This was of particular interest to the authors as it implied that N-WASP works downstream of the Tuba-Cdc42 pathway, prompting Otani to propose a scenario in which Tuba-Cdc42 activates N-WASP to enhance actin polymerization at the apical junction level. This results in the delivery of polymerized actin filaments to the lower portions of cell junctions. This area then serves as the dock to which the E-cadherin-catenin complex is anchored. A number of studies had implicated other GEFs, in addition to Tuba, as contributing to the cell-cell adhesion process.Revealing Tuba’s role in maintaining cellular morphology is an important step forward in understanding how different GEFs share cell assembly regulation roles - an intriguing topic and one that Otani and his colleagues look to tackle in the future. “We are now focusing on the role of Tuba in epithelial remodeling during morphogenesis,” says Otani. “This is in addition to adopting a cell biological approach to examine Tuba’s role in the organization of lateral actin filaments and its relationship with apical junctions.” |
|||||||||
|
|||||||||
 |
|||||||||
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |