| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
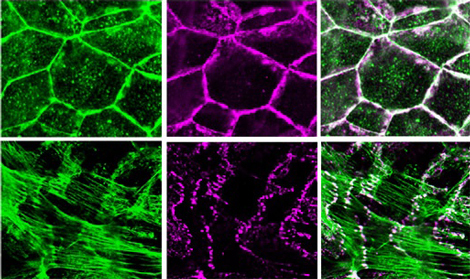
Recent work by Kentaro Abe of the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi; Group Director) now points to a role for the actin-binding protein EPLIN (Epithelial Protein Lost In Neoplasm) in bringing these adhesion and cytoskeletal apparatuses together. His findings are reported in the Proceedings of the National Academies of Sciences. Spurred by a 2005 study that showed no direct binding between the catenin-cadherin complex and actin, Abe and Takeichi initiated a screen for binding partners of α-catenin, one of the three catenin proteins (the others are β- and p120-catenin) that bind to cadherin’s cytoplasmic tail, in the hopes of identifying candidates for the missing link. Co-immunoprecipitation, a technique used to discover protein interactions, showed that EPLIN bound to α-catenin, and subsequent studies using mutants in which various domains of the EPLIN protein were deleted revealed that this interaction requires both the N- and C-terminal domains of EPLIN, and a protein-binding region known as VH3-C in α-catenin. The case for EPLIN association was further shored up by its localization within epithelial cells, which overlapped closely with cadherin, catenin and F-actin along lateral cell-cell contact sites. To test for EPLIN’s role in intercellular junctions, Abe next knocked down EPLIN expression by siRNA and found this had a number of dramatic effects: adhesion belt organization was disrupted, and cadherin showed up only in isolated points, rather than its usual continuous expression. Interestingly, junctional F-actin became radially organized, terminating at points of cadherin expression, while non-junctional actin was apparently unaffected. EPLIN is known to suppress actin depolymerization (the loss of actin molecular units from the ends of F-actin, the long, strand-like actin fibers), so Abe next tested whether this is important to the role played by EPLIN at the adhesion belt as well, and determined that it does stabilize F-actin, but that it does so independent of α-catenin. The association of catenin with F-actin, however, is highly dependent on EPLIN, as was shown by experiments in which beads coated with α-catenin that had either been treated or untreated with EPLIN were incubated with F-actins: only the treated beads pulled down F-actin. And, by adding EPLIN to the cadherin-catenin complex coated beads, they were able to reconstruct the cadherin-F-actin association, which was called into doubt by the 2005 study. Abe’s series of experiments has revealed a new function for EPLIN in linking the cadherin-catenin machinery to the actin cytoskeleton, but the fact that F-actin still radiates to bind cadherin at punctate cell contact points in EPLIN deletion mutants suggests that other molecules mediating actin-cadherin complex association still await discovery. The roles of the different protein binding VH domains in α-catenin may hold clues to this mystery. “Although the classic view of the cadherin–F-actin association was recently challenged, it was clear that cadherin always co-localizes with F-actin, which made the new story difficult for some to accept,” says Takeichi. “Our results could at least in part settle the controversy, by revealing a new mediator between cadherin and F-actin, which suggests that the old understanding was not so wrong after all. And of course there may yet be new stories to be uncovered, and so we will continue to dissect this mysterious molecular machinery.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |