| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
October 12, 2011 –Epithelial cells stay connected in a number of ways, relying on combinations of adhesion molecules to recognize and maintain contact with their neighbors. One form of epithelial cell-cell adhesion involves the zonula adherens, a belt-like region at the cell border at which complexes of cadherin, catenin and other molecules, including actin filaments in the cytoskeleton, accumulate. Sheets of epithelial tissue undergo rearrangements in the course of numerous developmental and disease processes, ranging from convergent extension to wound closure. In the latter, purse-string-like actomyosin cables linked to adherens junctions (AJs) contract around the leading edges of cell margins, closing the space between cells, and are known to. It is not known, however, how actin cooperates with the cadherin machinery in the remodeling of the AJ.
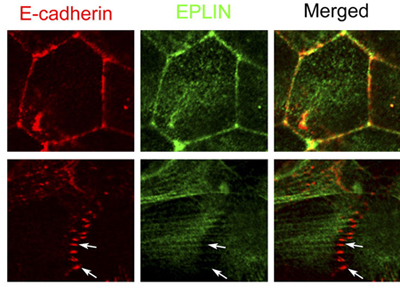
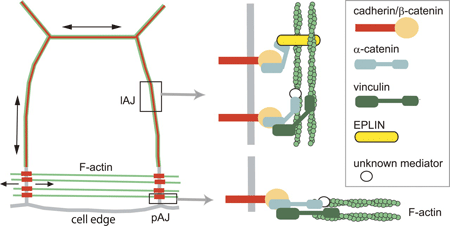
New work by Katsutoshi Taguchi and others in the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi, Group Director) shows that the protein EPLIN plays a key role in the process, as a mechanosensitive regulator of junctional remodeling. Published in the Journal of Cell Biology, this research sheds new light on how physical forces, such as stretching, help determine cellular properties and behaviors. Taguchi began by immunostaining a line of cultured epithelial cells for E-cadherin and F-actin, showing their accumulation at adherens junctions. Interestingly, this labeling revealed that these junctions took two different forms. In the interior of a cell colony, the proteins aligned linearly, whereas at the fringes, the junctions were punctate in form. These junction types appeared to be interconvertible, as both forms could be identified within single colonies. In both the linear and the punctate adherens junctions, E-cadherin, β- and αE-catenins, and actin fibers play dynamic structural roles. Several proteins, including EPLIN and vinculin, have been identified as linkers between the cadherin-catenin complex and the actin cytoskeleton to date, prompting Taguchi to examine their possible roles. Through additional immunostaining, he found that while vinculin is present in both linear and punctate AJs, EPLIN was only detectable in the linear type. Knockdown of the two proteins by RNAi further supported a role for EPLIN; loss of the function of this protein allowed the formation of punctate AJs only, suggesting that it is required in the formation of the linear adherens junction. What then is the reason for the lack of EPLIN at punctate AJs? The group focused on F-actin as a candidate. In linear AJs, actin fibers run parallel to the cell membrane, while in the punctate they are perpendicular, suggesting a possible influence by mechanical forces such as tension in realigning the cytoskeleton. Taguchi tested this hypothesis by laser ablating points of punctate AJs targeted by F-actin. Loss of this tension-generating structure resulted in immediate upregulation of EPLIN as well as E-cadherin, and the adherens junctions converted to the linear form. In the converse experiment, when parallel actin fibers were ablated from linear AJs, the EPLIN signal was lost, suggesting that this molecule responds to “pulling” forces on cells by accumulating at linear junctions. A subsequent test of this idea using a stretching apparatus to exert tension on epithelial colonies further showed that this stimulated the localization of EPLIN at the cell-cell junction.
The current study provides new insight into how EPLIN plays a mechanosensory role in the remodeling of adherens junctions in response to mechanical forces. The punctate adherens junction may play a stabilizing role by giving flexibility to otherwise rigid sheets of epithelium under mechanical stress. “Many types of epithelial tissue are subject to external mechanical stimuli, but they tend to be quite resilient and remain intact even when pulled and stretched,” says Takeichi. “It seems that these sheets of cells must have some mechanism for detecting and responding to mechanical stresses. It will be intriguing to explore whether the function of EPLIN observed in vitro in this study plays roles in vivo as well.” | |||||||
|
|||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |