| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
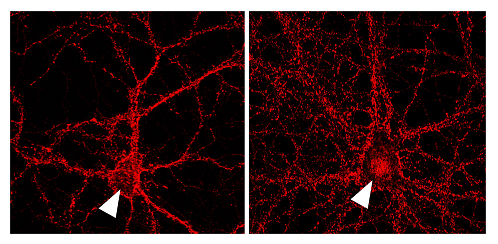
Abe found that after NMDA-R activation, calpain cleaves the N-terminus of the β-catenin protein, protecting it from degradation within the cytoplasm. Ordinarily, full-length β-catenin is phosphorylated by the kinase GSK3b, marking it for ubiquitination and digestion in the proteasome. Activation of the Wnt pathway protects against this degradation, allowing β-catenin to make its way into the nucleus, associate with the transcription factor Tcf/Lef, and activate the transcription of various genes. Interestingly, β-catenin cleaved by calpain is also safeguarded from destruction in the cytoplasm and thus able to reach the nucleus and engage transcription. “While the evidence is not yet conclusive, it appears possible that calpain even cleaves β-catenin that is bound to cadherin, which, if it proves to be true, would set up a link between neuronal activity and a loosening of the adhesion between cells at the synapse,” notes Abe. Additional tests confirmed that this cleavage of β-catenin could be blocked by the administration of a calpain inhibitor, and its effects on Tcf-dependent gene transcription could be mimicked by the transfection of β-catenin fragments of the same length. Abe next turned to the question of what genes might be activated by this pathway, and on analyzing transcripts amplified by RT-PCR, finding that Fosl1, a gene also activated by the canonical Wnt pathway, was upregulated after NMDA-R stimulation by glutamate (which leads to calpain activation). Again, this effect was blocked by calpain inhibition and duplicated in experiments using exogenous β-catenin fragments, strongly indicating that a novel pathway in which NMDA-R induces calpain to cleave β-catenin was being used to initiate the transcription of genes in hippocampal neurons. Looking ahead, Abe says, “For the next step, I’d like to try to work out what role this transcriptional activity plays in nervous system functions such as memory and learning. And, of course, a possible scenario in which neuronal activation sets off interactions between cellular adhesion and transcriptional pathways remains intriguing.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |