| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
Nov 12, 2012 –The cornea of the eye serves a dual role, protecting the sensitive interior tissue from the environment and supporting the lens by focusing incoming light. With a thickness of only 0.5 mm in humans, the cornea nonetheless has a complex stratified structure, including epithelial, stromal, and endothelial layers. The innermost of these, the endothelium, separates the corneal stroma from the aqueous humor of the eye’s interior, acts as a point of entry for nutrients into the non-vascular overlying tissue, and as a pump that siphons away excess fluids from the corneal interior. Defects in endothelial function can thus lead to accumulations of fluid that cloud the otherwise transparent cornea, leading to blurring of vision. Although it is known that this selectively permeable barrier is created by junctions between endothelial cells, the molecular mechanisms behind it have remained unclear.
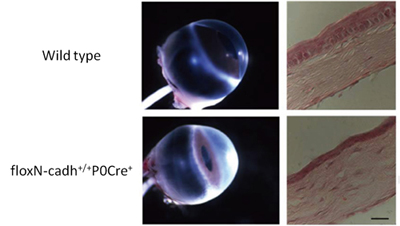
A new study by Vassil S. Vassilev in the Laboratory for Cell Adhesion and Tissue Patterning (Masatoshi Takeichi, Group Director) and others in CDB now show that N-cadherin plays an important role in maintaining corneal integrity. In an article published in Investigative Ophthalmology and Visual Science, the group shows that loss of N-cadherin in mouse leads to defects in endothelial cell junctions, leading to stromal edema and a range of other defects in the cornea. Corneal endothelial cells are bound to their neighbors in monolayers by two forms of cell-cell adhesion: tight junctions and adherens junctions, which together are known as the apical junction complex. Tight junctions are established by multiple types of adhesion molecule and form a physical barrier between cells, while adherens junctions are formed by classic cadherin adhesion molecules, which work to stabilize the links between adjacent cells and to regulate cell morphology through interactions with the cytoskeleton. In the cornea, the N-cadherin gene is highly expressed in the fetal through adult stages, which led the group to investigate the exact role of this molecule in corneal function. Vassilev began by generating a conditional knockout mouse in which the N-cadherin gene was deleted specifically in neural crest cells, the main embryonic source of the corneal endothelium. After confirming that N-cadherin expression was lost in mutant corneal endothelial junctions, the group next examined the tissue structure and found it to be much more opaque than wildtype cornea. Interestingly, although N-cadherin was selectively ablated from the endothelium, the effects of its loss were seen in the other corneal layers. In the stromal layer, collagen fibrils became unevenly spaced and fluid accumulated, resulting in edema, while the epithelium grew thinner and exhibited delamination and abnormal apoptosis. But how does loss of an endothelial cadherin perturb the tissue architecture of other corneal layers? Suspecting the answer might lie in (fine) structural changes of endothelial layer, Vassilev et al. compared cytoskeleton of mutant and unmodified cells by staining for F-actin (which connects with cadherins via catenin linker molecules). In wildtype endothelium, actin molecules ring the cells’ circumference, maintaining the structure of cell-cell junctions. In the conditional N-cadherin knockouts, however, the actin cytoskeleton was malformed, leading to defects in nuclear morphology and cellular adhesion. Additionally the group discovered defects in endothelial tight junctions, finding that expression of the tight junction marker ZO-1 was significantly downregulated and the junctions disorganized. Tests of the ion pumping function of mutant endothelial cells further revealed that their ability to transport sodium and water across endothelium layer was disturbed. This suggested loss of the endothelial barrier function leading to abnormal distribution of electrolytes and the excessive accumulation of fluids in the corneal tissue. “In vitro studies had suggested that cadherin-based adherens junctions can be important for the maintenance of tight junctions, but here we were able to show that this is the case in vivo as well,” says Takeichi. “In this study, we showed how the adherens junction plays a critical role in the exquisite architecture that gives the cornea its transparency. We next hope to use this conditional knockout system to investigate the function of N-cadherin in other neural crest-derived tissues.” |
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |