| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
April 19, 2010 – One of the main functions of somatic (or adult) stem cells is to keep the body’s systems in working order by generating replacements for cells that have been damaged, or simply aged through normal wear and tear. As the body grows older, however, its stem cells gradually lose their ability to self-renew through cell division, a phenomenon known as cell senescence. A number of cell cycle mechanisms have been linked to this form of cellular aging, but its molecular basis remains poorly understood. A recent report by Yuki Kujuro and others in the Laboratory for Cell Lineage Modulation (Toru Kondo; Team Leader) identifies a new genetic inducer of cell senescence in the nervous system. The gene, Ecrg4, encodes a secreted protein that triggers the degradation of cell cycle factors in oligodendrocyte progenitor and neural precursor cells, marking it as an important link in the aging of the brain.
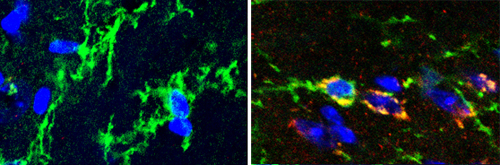
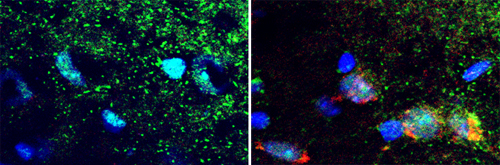
Kujuro began by observing the aging process in oligodendrocyte progenitor cells (OPCs), and found a number of irreversible changes in their morphology and internal regulation that take place when they are cultured in a high concentration of serum, which serves as an experimental surrogate for in vivo aging. Looking next at changes in gene expression, she found more than a thousand were up- or down-regulated on high serum culture, more than three hundred of which remained in that state even after the culture medium was switched back to a normal serum level. Among the genes caught in this screen, Ecrg4, caught the team’s interest for several reasons. The difference between its expression levels in senescent and non-senescent was greater than for any other gene, and its expression was also activated in senescing mouse embryonic fibroblasts, shown for the first time in this report. In addition, its expression is down-regulated in tumors, which are notorious for their age-defying ability to self-renew indefinitely. To test for function, the team transfected rat OPCs with Ecrg4 and watched for signs of senescence. Compared to control populations, the transfected cells showed significantly higher activation of senescence markers. Subsequent experiments showed that the gene’s effect is likely achieved through a combination of the degradation of cell cycle factors, cyclin D1 and D3, and dephosphorylation of Rb. Having established a solid case for the involvement of Ecrg4 in OPC senescence in vitro, Kujuro next looked at the mouse brain to see if it had similar effects in vivo. She found that while the gene showed only weak expression in the brains of young mice, it was much higher in aged brain, specifically in the regions where OPCs and neural precursor cells reside. Interestingly, Ecrg4 was upregulated even in terminally differentiated neurons, which have ceased dividing and should therefore be immune to senescence. “The question of ‘Why do we age?’ is a longstanding mystery, and since the discovery of stem cells, people have speculated that it may be due in part to natural wear and tear affecting their ability to function,” says Kondo. “In this study, we identified one mechanism relating to the aging of neural stem and progenitor cells, and we’re hopeful that this will lead us to a better understanding to diseases of the nervous system.”
|
|||||||
|
|||||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |