| RIKEN Center for Developmental Biology (CDB) 2-2-3 Minatojima minamimachi, Chuo-ku, Kobe 650-0047, Japan |
June 22, 2012 –Chromosomes in eukaryotic cells contain regions of compactly bundled DNA and protein called heterochromatin, which serve as loci for the epigenetic regulation of gene expression, frequently in the form of gene silencing. In a sense, genes in heterochromatic regions are “locked away” from transcription factors that might trigger their expression. Recent studies in the fission yeast, Schizosaccharomyces pombe, have shown that the RNAi machinery is involved in heterochromatin assembly in centromeres, but direct mechanistic links between these two processes have remained elusive. New work by Mayumi Ishida and colleagues in the Laboratory for Chromatin Dynamics (Jun-ichi Nakayama, Team Leader) has now shown how the RNAi factor Chp1 contributes to heterochromatin assembly and gene silencing through direct binding not only to methylated histones but to DNA and RNA as well. Published in Molecular Cell, this new report provides new mechanistic insights into this crucial genetic regulatory system.
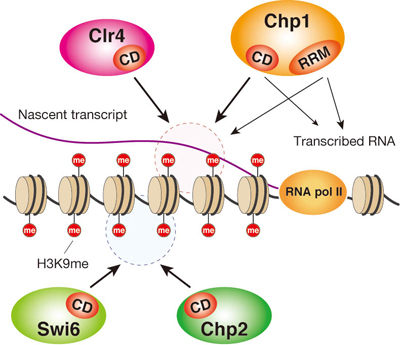
The construction of heterochromatin regions relies on a process called histone methylation, in which DNA-associated histones are decorated with methyl residues as a kind of identifying label. In fission yeast, a methyltransferase called Clr4 methylates the ninth lysine of histone H3 (called H3K9me), which then binds HP1 proteins (such as Swi6 and Chp2) resulting in the formation of a transcriptionally silent chromatin region. RNAi, in contrast, is a mechanism by which targeted RNAs are degraded post-transcriptionally. The centromeric regions of fission yeast chromosomes express non-coding RNAs at low levels; after their transcription, these are cleaved into double strands by RNAi machineries and enzymatically diced to short sequence fragments known as siRNAs. These ribonuclear snippets form part of a larger complex called RITS, which itself binds to chromatin by targeting of these non-coding RNAs. This elaborate process is known to recruit Clr4 to the centromere and induce histone methylation. With this as a background, the Nakayama lab’s most recent study focused on Chp1, a component of the RITS complex, which plays a critical role in tethering RITS to the centromere. Notably, the Chp1 protein structure features a region known as a chromodomain (CD), which its shares with other CD proteins such as Clr4, Swi6, and Chp2. Chp1 also includes a different domain called RRM, which is known as an RNA binding site in other proteins, leading them to ask whether it would have the same function here. On conducting an electrophoretic binding assay to answer this question, they were surprised to find not only that RNA was bound not only by RRM, but by the chromodomain as well. This effect was intensified nearly ten-fold by histone methylation, which also enabled DNA binding by Chp1, suggesting that the binding of this protein to chromatin is stabilized via a CD-mediated interaction with H3K9me. Interesting, but was this discovery important in vivo as well? Ishida generated Chp1 mutants to test for effects on chromatin structure and found that defects in either the methylated histone- or the nucleic acid-binding domains resulted in a dramatic reduction in localization of Chp1 to the centromere, and a concomitant drop in heterochromatin function. The team will be focusing next on whether this activity is unique to Chp1, or more widely shared by its chromodomain protein kin. “Our next step will be to look into whether the nucleic acid-binding function of Chp1 is at work in other heterochromatic regions, and in other eukaryotes,” says Nakayama. “This will be an important part of our broader focus on unraveling the roles of CD proteins in general in heterochromatin assembly and RNAi.” |
|||||
|
|||||
|
|||||
 |
| Copyright (C) CENTER FOR DEVELOPMENTAL BIOLOGY All rights reserved. |