




     |

 There
can be few, if any, cells as specialized as sperm and egg; they appear
unique (you can even see a mammalian oocyte with the naked eye) and don't
typically divide by themselves. Yet when mammalian sperm and egg combine
at fertilization, the single cell they generate is transformed within
hours to produce a totipotent cell: one which is completely unspecialized
in that from it all cell types develop to produce an entire individual.
There
can be few, if any, cells as specialized as sperm and egg; they appear
unique (you can even see a mammalian oocyte with the naked eye) and don't
typically divide by themselves. Yet when mammalian sperm and egg combine
at fertilization, the single cell they generate is transformed within
hours to produce a totipotent cell: one which is completely unspecialized
in that from it all cell types develop to produce an entire individual.
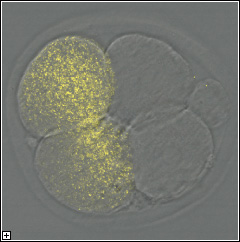 Our
laboratory combines molecular and cell biology with piezo-actuated micromanipulation
of mouse gametes and embryos to study the events that occur immediately
after sperm-egg union (oocyte activation) and their developmental consequences.
One long-standing question concerns why oocytes don't begin to divide
by themselves in the absence of a sperm. To address this, our group has
developed a novel approach that yielded the revelation that the removal
of a protein, which we dubbed Emi2, caused oocytes to progress through
the cell cycle without stopping, as if the oocytes had been activated
by a sperm. We then extended this to show that Emi2 works through Cdc20
and that both parthenogenetic activation and fertilization require Cdc20.
This may be the first formal demonstration that the signaling responsible
for MPF ablation has molecular components common to both parthenogenesis
and fertilization, which is significant given the application of parthenogenetic
activation in nuclear transfer and other current research.
Our
laboratory combines molecular and cell biology with piezo-actuated micromanipulation
of mouse gametes and embryos to study the events that occur immediately
after sperm-egg union (oocyte activation) and their developmental consequences.
One long-standing question concerns why oocytes don't begin to divide
by themselves in the absence of a sperm. To address this, our group has
developed a novel approach that yielded the revelation that the removal
of a protein, which we dubbed Emi2, caused oocytes to progress through
the cell cycle without stopping, as if the oocytes had been activated
by a sperm. We then extended this to show that Emi2 works through Cdc20
and that both parthenogenetic activation and fertilization require Cdc20.
This may be the first formal demonstration that the signaling responsible
for MPF ablation has molecular components common to both parthenogenesis
and fertilization, which is significant given the application of parthenogenetic
activation in nuclear transfer and other current research.
In addition, we are interested in the interactions between sperm head components and the oocyte, in a bid to discover what happens during fertilization and the earliest moments of the new embryo. It would be useful to attribute molecular identities to the proteins involved in these interactions and characterize them functionally. This task is a daunting one, as beneath the membrane of a sperm head reside macromolecular assemblies that include a nucleus (containing the paternal genome and associated proteins) and a surrounding cytoplasmic matrix, the perinuclear matrix, which rapidly comes into contact with the oocyte cytoplasm at fertilization and is an immediate source of paternally-contributed molecules that may modulate development. With a greater understanding of this development, our lab hopes to gain insights into the processes by which embryonic stem cells are formed and carcinogenesis is initiated.