- Overview
- Project 1: Polarity
- Project 2: Junctions
- Project 3: Invagination
- Project 4: Reconstruction
- Project 5: Reversibility
Overview
The central question in developmental biology is how cells, tissues and organs acquire their specific functions and distinct shapes. A large body of work over the past several decades has yielded a broad understanding of how differential gene expression establishes functional specialization of cells and tissues. In contrast, far less is known about how cell shapes and tissue structures are controlled and remodeled. Although a simple view has emerged whereby cytoskeletal dynamics plays a central role in determining the cell shapes, and individual cell shape changes collectively contribute to the construction of overall tissue architecture, the underlying molecular and mechanical mechanisms remain largely unexplored. We aim at identifying novel cellular mechanisms that control cell shapes during epithelial morphogenesis. We also seek to understand how local cell shape changes mechanically influence the neighboring cells to sculpt the overall tissue structure. Our overall goal is to comprehensively understand the molecular and mechanistic principles that govern tissue morphogenesis in order to conceptualize the origin and evolution of morphological diversity both within an organism and between closely related species that display distinct processes of tissue morphogenesis.
 |
Fig. 1 Animal development consists of two general types of events: pattern formation and tissue morphogenesis. |
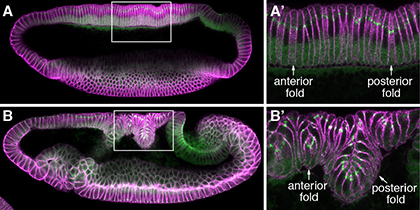
We are currently investigating several novel mechanisms that initiate and shape the two epithelial folds that form during the early embryonic development of fruit flies, Drosophila melanogaster. Our previous work identifies changes in the apical-basal polarity and repositioning of adherens junctions in two narrow strips of dorsal epithelial cells as a non-canonical mechanism for the initiation of epithelial folding. We subsequently found that although these two epithelial folds undergo similar initiation process, their ultimate depths differ and relate to the differing strengths of physical coupling between adherens junctions and their underlying cytoskeleton. Our research combines cutting-edge imaging technologies with a variety of Drosophila genetic tools to try to understand how modifications in epithelial cell polarity control cell shape and how local mechanical processes determine global tissue architecture.
|
Fig. 2 Dorsal fold formation |
In addition to these main lines of research, we are pursuing several collaborations to develop new analytic tools for, and to conceptually broaden our horizon on, the general questions concerning epithelial morphogenesis. One ongoing project is a long-term cross-disciplinary collaboration with computer scientist Zia Khan at University of Maryland, College Park, in which we aim at developing 4D image processing tools that quantitatively analyze geometric and structural changes during dynamic epithelial morphogenesis. We have also begun collaboration with evolutionary developmental biologist Steffen Lemke on the evolutionary history of emergent morphogenetic structures that are unique in certain evolutionary lineages. We seek to understand the function of these prominent, yet transient morphological structures that do not eventually contribute to an organ or body part in the adult animal.
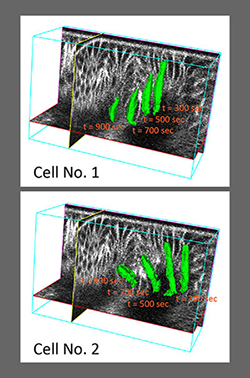 |
Fig. 3 EDGE4D: image processing, 3D cell shape rendering and cell tracking. |
Project 1: Polarity sculpts the shape
How do localized changes in cell polarity organize the bending of an epithelium?
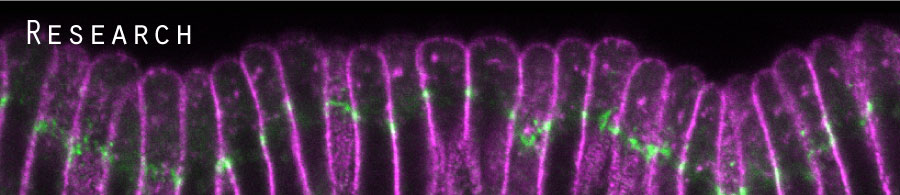
The formation of complex epithelial structures is often driven by changes in cell shapes. In most epithelial folding events that have been examined so far, the initial cell shape changes are associated with reorganization of cytoskeletal network at the cell cortex resulting from contraction of actin network under the control of the activated form of the molecular motor protein non-muscle myosin. We recently, however, identified a novel mechanism for the initiation of epithelial folding that is distinct from the myosin-dependent mechanism. Specifically, we found that epithelial folding depends on post-translational downregulation of an evolutionary conserved polarity protein called Par-1 during the formation of two epithelial folds during Drosophila gastrulation. Although cell polarity is critical for the functional specialization of a variety of cell types and underlies the geometric patterning of many tissues during animal development, our observation was the first that links differential modifications of cell polarity to anisotropic cell shape changes along the epithelial apical-basal axis that initiates tissue bending. Conceptually speaking, our data suggest a possibility whereby differential modulation of cell polarity across the tissue introduces mechanical imbalances across the tissue that organizes the folding of epithelial tissues.
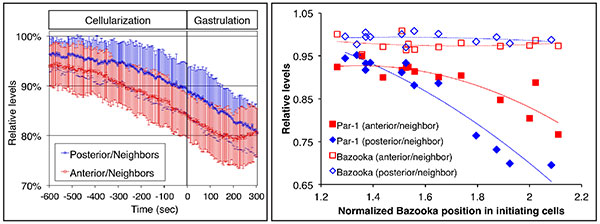
Fig. 1 Downregulation of Par-1 during the initiation of dorsal fold formation.
The
left panel shows quantitation of the levels of Par-1 in the initiating cells relative to those
in the neighboring cells as a function of time. Par-1 levels are monitored using live
imaging on GFP-tagged Par-1. Par-1 levels decrease about 15% before the onset of
gastrulation. The right panel shows quantitation of junctional positioning based on
Bazooka positioning and the levels of Bazooka and Par-1 in the initiating cells. While the
relative levels of Bazooka remain constant, the relative levels of Par-1 decrease in cells
in which junctions are positioned more basally.
Apical-basal polarization is a defining feature of the epithelial tissues. Epithelial folding resulting from a non-uniform landscape of the epithelial polarity could potentially be a general mechanism that is widely used. Understanding how Par-1 is downregulated holds the key to understanding the spatial information that organizes this epithelial folding event. Moreover, since Par-1 is an evolutionarily conserved serine/threonine protein kinase that has numerous substrates. Identifying the relevant Par-1 substrate(s) that controls the cell shape will be crucial to understanding the molecular mechanisms underlying the polarity-based cell shape changes and epithelial remodeling.
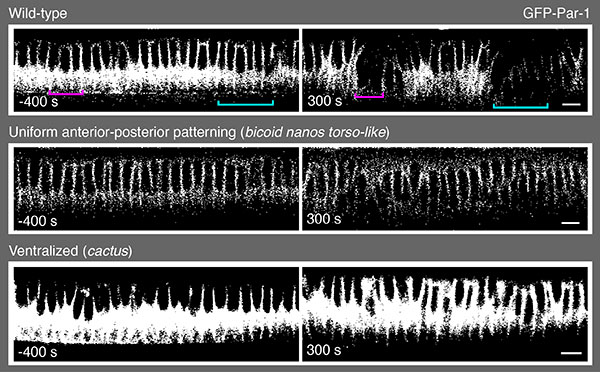
Fig. 2 Par-1 downregulation requires positional information specified by
anterior-posterior and dorsal-ventral patterning systems.
In contrast to the wild-type
embryo in which Par-1 becomes downregulated in the initiating cells, embryos that do
not possess anterior-posterior patterning information (bicoid nanos torso-like mutants) or
embryos that are ventralized (cactus mutants) display uniform levels of Par-1.
We are interested in the following questions:
1) How does the early embryonic patterning system provide the spatial and temporal information for Par-1 downregulation? We will genetically dissect the gene regulatory circuits that provide such spatio-temporal information.
2) What is the molecular mechanism that underlies the process of Par-1 dwnregulation? Since Par-1 downregulation likely involves post-translational modification and degradation, we will perform structural-functional analysis to identify domain(s) in the protein Par-1 that is responsible for the downregulation.
3) How does Par-1 downregulation leads to cell shape changes? We will use a proteomic approach to catalog potential Par-1 substrates to search for the relevant substrate(s) that regulates cell shape changes.
4) How does Par-1 molecularly control the positioning of adherens junctions through its interaction with the PDZ domain containing scaffolding protein Bazooka (the Drosophila homolog of Par-3)? We showed previously that downregulation of Par-1 leads to basal shift of adherens junctions, a crucial event that triggers the cell shape change. See Project No.2 for details.
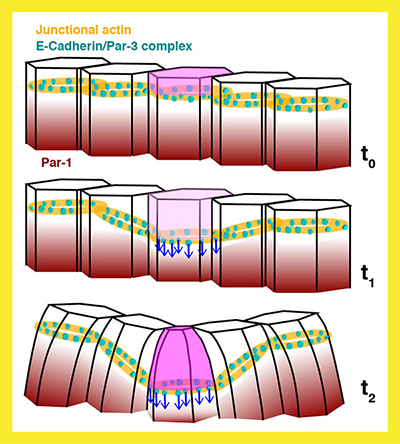 |
Fig. 3 Schematic model for the initiation of dorsal fold formation under the control
of Par-1-based polarity change. |
Project 2: Shifting (junctional) positions
How does basal repositioning of adherens junctions lead to cell shape changes?
We previously identified the basal shift of the positioning of adherens junctions as a crucial event that initiates cell shape changes. Prior to the cell shape changes, adherens junctions reposition toward the basal side of the cells, apparently responding to an increase in the ratio between the scaffolding protein Bazooka and Par-1 as Par-1 becomes downregulated. Since Bazooka is a known Par-1 substrate and localized exclusively in the junctional region where it directs the assembly of adherens junctions, understanding how changes in Bazooka/Par-1 ratio controls the positioning of adherens junctions holds the key to understanding how changes in cell polarity lead to the alteration of cell shapes. Moreover, while the basal repositioning of adherens junctions is followed by anisotropic cell shape changes along the apical-basal axis -- the apices of cells shrink to eventually shorten the cell height, it remains unclear how basal shift of junctions actually induce apical cell shape changes. One provocative idea is that the shift of the junctional positioning creates a mechanical imbalance between apical and basal-lateral membrane surfaces that have differing mechanical properties. This model implies that junctions define the boundaries between two distinct membrane surfaces, representing a novel function that is distinct from its canonical, structural function of maintaining epithelial cohesion and integrity. Our goal is to address the following questions.
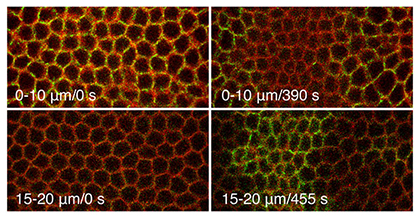 |
Fig. 1 Adherens junctios in the initiating cells shift basally during dorsal fold
formation. |
1) Does the junctional shift involve rapid cycles of junctional turnover? We will estimate the rate of junctional turnover using fluorescent recovery after photobleaching or FRAP during junctional shift.
2) Does the junctional shift involve mechanical influences exerted by the cytoskeleton networks? Both actin filaments and microtubules associate with adherens junctions and it is possible that the basal shift is propelled not by the rapid cycles between assembly and disassembly, but by the puling or pushing of the junctional complexes by the cytoskeletons themselves or their associated motor proteins. We will perturb actin and microtubule networks and examine key regulators of their dynamics to test this idea.
3) Can we probe the mechanical conditions of the apical and basal-lateral membrane surfaces during the junctional shift? As mentioned above, such measurements could provide important clues as to how basal shift of junctions result in apical cell shape change. We will use laser ablation to reveal the levels of tension that is present within the apical membrane and we will construct fluorescence resonance engergy transfer or FRET based tension sensor to visualize the cortical mechanical landscape across the tissue.
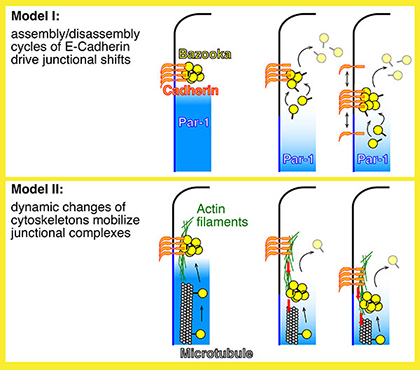 |
Fig. 2 Two mechanistic hypotheses for the basal shifts of junctions. |
Project 3: (Un)coupled and invaginate
How does the strength of physical coupling between adherens junctions and the actin networks control the degree of invagination?
The folding of an epithelial tissue begins with spatially restricted cell shape changes of the initiating cells that initiates the bending of the tissue and typically continues with invagination of the cells that neighbor the initiating cells. Because the neighboring cells do not initially display cell shape changes, their invagination has long been thought of as a passive response to the driving forces that the initiating cells generate. The two epithelial folds that we have been investigating initiate much the same way with respect to the downregulation of Par-1, the basal shift of junction positioning and the initial cell shape changes. However, the two epithelial folds undergo distinct degrees of invagination to become very different in their final lengths. Therefore, it appears that distinct properties or even active processes that are associated with the neighboring cells may control the extent of neighboring cell invagination. Our experimental system thus provides a unique opportunity whereby the molecular and mechanical mechanisms that govern the process of neighboring cell invagination can be examined.
Our previous work sought to understand how the differing invagination behaviors are regulated. We unexpectedly observed that in embryos that have reduced activity of α-Catenin both epithelial folds undergo extensive invagination. Since α-Catenin functions as a molecular bridge that couples the core components of adherens junctions to the underlying actin network, our data suggest that reduced coupling between junctions and actin promote invagination, whereas tight coupling restricts invagination. Although previous work indicated that in most contexts loss of α-Catenin activity results in a loss of junctional stability and disintegration of the epithelial tissues, increasing evidence suggests that α-Catenin has additional morphogenetic function beyond ensuring the integrity of cell-cell adhesion. Moreover, we showed that distinct activity states of the small GTPase Rap1 act through a α-Catenin-dependent process to confer differing degrees of neighboring cell invagination. Thus, we are interested in the following questions.
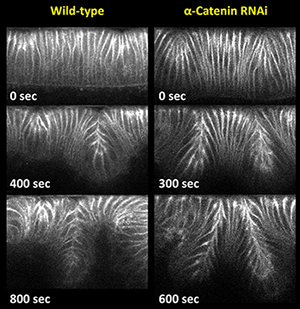 |
Fig. 1 Loss of α-Catenin promotes extensive invagination. |
1) How does Rap1 influence α-Catenin’s ability to physically couple junctions to the actin cytoskeleton? This question relates to the structural and functional feature of α-Catenin. We plan to first identify regions in the α-Catenin protein that confer distinct invagination phenotypes. This information will help us to seek the Rap1 effector(s) that may regulate the α-Catenin-dependent junction-actin coupling.
2) How does α-Catenin-dependent junction-actin coupling influence the cell biological and physical properties of the neighboring cells to control the degrees of invagination? We plan to develop an imaging strategy to visualize the coupling process using a FRET-based sensor. We will also use quantitative 4D imaging analysis (see Project No.4) to extract geometric features of the neighboring cells as they resist or participate in the process of invagination.
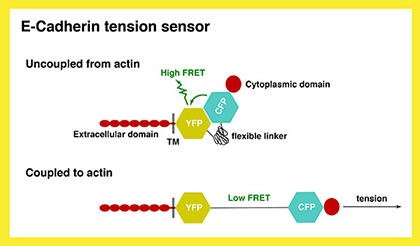 |
Fig. 2 Schematic representation of the E-Cadherin tension sensor. |
Project 4: Edging in the 4th dimension
Developing strategies and software tools for quantitative 4D imaging (with Zia Khan, University of Maryland, College Park)
Quantitative 3D analyses of cellular dynamics using time-lapse microscopy data have the potential to greatly advance our understanding of complex morphogenetic processes. Yet, obtaining these measurements remains technically challenging due to the intrinsic noise in these data. In collaboration with computational scientist Zia Khan, we previously developed an imaging processing software that allows us to automate segmentation, 3D rendering and quantitative analyses of geometric features of cell shapes, positioning of adherens junctions and protein concentrations at single cell resolution. This tool enables us to perform 3D quantitation on image stacks generated by confocal microscopy using fixed samples that have been labeled with immunofluorescence.
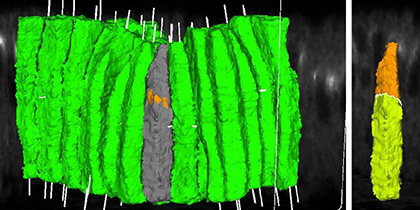 |
Fig. 1 3D rendering of cell shapes and junctional positioning during dorsal fold
initiation. |
Epithelial morphogenesis is, however, a dynamic process that information on the 4th dimension – the time dimension – is absolutely critical. Therefore, we included the tracking functionality of our existing 3D image processing program and improved its overall performance with the addition of several novel algorithmic strategies, such as techniques that leverage the duality between surface meshes and binary image volumes. The result is EDGE4D, a software tool that allows reliable, systematic, and dynamic analyses of a large number of densely packed membrane-labeled cells, analyses that were previously unattainable by existing methods.
We continue to improve the robustness and accuracy of EDGE4D performance from both the imaging angle as well as the computational angle. This is an ongoing and long-term collaboration that has already and will continue to enhance our ability to perform and automate quantitative live imaging analysis for epithelial morphogenesis.
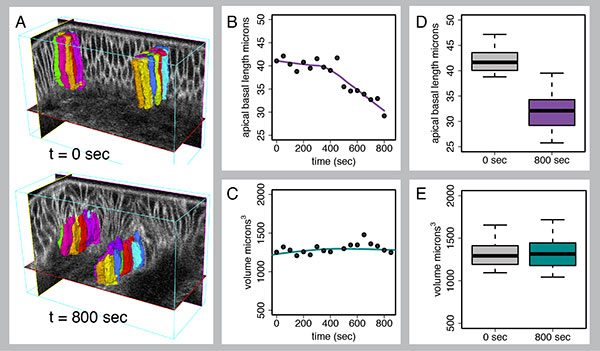
Fig. 2 Quantitative 4D analysis of dorsal fold formation using EDGE4D.
This figure
analyses the height and volume of the initiating cells during dorsal fold formation. Z
stack, time-lapse images of dorsal fold formation were first produced using two-photon
microscopy on embryos expressing a membrane and a nuclear marker. The 4D image
stack was then processed with EDGE4D to identify, reconstruct and track the initiating
cells (A). Cell height was measured that confirmed our previous observation that the
initiating cells undergo substantial shortening (B, D). In contrast, cell volume remains
constant (C, E), indicating that the initiating cells obey the principle of volume
conservation during anisotropic cell shape changes.
Project 5: Beautiful but short-lived
The Developmental function and evolutionary emergence of transient morphogenetic structures (with Steffen Lemke, University of Heidelberg, and Zia Khan, University of Maryland, College Park)
Tissue morphogenesis produces complex structures that ultimately contribute to organs and body parts. However, certain morphogenetic events produce prominent morphological structures that are present only transiently and ultimately revert back to the original tissue architecture. The initial formation of these structures and their eventual reversion and disappearance raise several important questions: why do these structures form in the first place, and what and whether at all do these structures serve any function? If a structure is present transiently and does not contribute to an organ or body part, how did it emerge and become stabilized during the course of evolution? Moreover, if one considers the process of morphogenesis a change from a ground state for a tissue to a more advanced state, conceptually analogous to the process during which progenitor cells differentiate into a committed cell fate, could the reversion of morphogenetic states be considered a kind of “dedifferentiation” of tissue states. If so, what cellular and mechanical properties define the ground and the advanced states and what molecular characteristics permit such reversion of tissue states? These questions highlight the challenge to conceptualize this kind of morphogenetic events and suggest that something fundamental is missing in our understanding of animal developmental and evolutionary.
The cephalic furrow is one such structure that first forms prominently as a deep epithelial fold, but subsequently reverts back to the original flat state of tissue architecture. Unlike the other two morphological landmarks that form during Drosophila gastrulation, the ventral furrow and the posterior midgut, which give rise to mesodermal and endodermal structures respectively, the cephalic furrow does not contribute to an organ or body part. It is thus seemingly paradoxical that the formation of cephalic furrow is highly stereotyped, implying that the process might have been shaped and maintained by strong selection forces during the course of evolution and that it must serve an important function. The molecular mechanisms underlying the formation of cephalic furrow is not known in spite of its frequent use as a pronounced spatial readout for early embryonic patterning and a temporal marker for the onset of gastrulation. Several hypotheses have been put forth as to what function cephalic furrow might serve. However, none of these hypotheses has been critically examined.
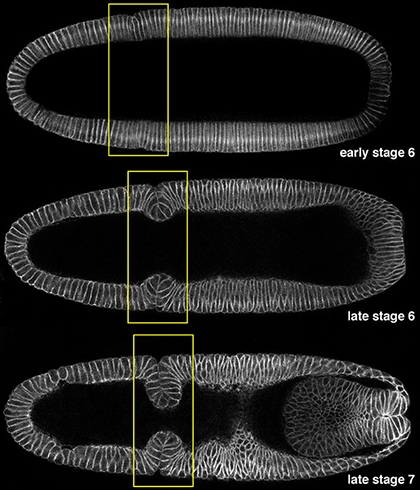 |
Fig. 1 Cephalic furrow formation. |
Our goal is to combine comparative genomics and quantitative live imaging approaches to address the mechanistic, functional and evolutionary questions concerning the formation of cephalic furrow. We are interested in the following issues.
1) What is the molecular mechanism that underlies the initiation of cephalic furrow formation? We will use live imaging approach to define the cellular changes that precede cephalic furrow cell shape changes. We will also perform cell-type specific transcriptome analysis to identify genes that are expressed specifically in the initiating cells in hope to identify cellular factors that initiate and orchestrate cephalic furrow formation. We aim at establishing a general mechanistic understanding for the formation of cephalic furrow.
2) When did the cephalic furrow emerge in the history of Dipteran evolution? Classical insect embryology indicates that insects of the lower branches do not form cephalic furrow. Our preliminary data suggest that certain lower Dipterans also do not form cephalic furrow. We will perform an embryological survey of several representative species from major lineages in the Dipteran phylogeny to define the genesis and the evolutionary pattern of cephalic furrow morphogenesis. We will examine whether the molecular process that underlies cephalic furrow formation in Drosophila is conserved among Dipterans that form cephalic furrow.
3) What is the developmental function of cephalic furrow? A potentially unbiased approach for the investigation of cephalic furrow functionality is to perform quantitative imaging to monitor cell shape changes and global tissue dynamics during early gastrulation in the region that surrounds the cephalic furrow. Such approach has become possible with the advent of in toto live imagine using light sheet microscopy. We will compare the wild-type data to those from embryos wherein the cephalic furrow is genetically ablated. We will also extend this analysis to representative Dipteran species where live imaging is possible so that we can compare the tissue dynamics between species that do and do not form the cephalic furrow.
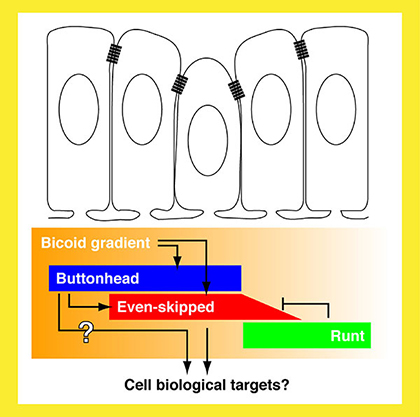 |
Fig. 2 The genetic circuit that underlies cephalic furrow initiation. |