
News and Announcements from the CDB
The mammalian skin is an important multifunctional organ with roles in sensation, thermoregulation and metabolism, in addition to protecting the body from the external environment. It is organized into a complex layered structure of epidermis, dermis and hypodermis, with each layer made up of a mix of cell and tissue types. These cells and tissues use intercellular communication to guide morphogenesis, as well as maintenance (or regeneration) of the skin. The synchronicity between the growth cycle of hair follicles (HFs) and the fluctuation in the thickness of the dermal adipocyte layer (hypodermis) is one model of intercellular communication in the skin. The hypodermis increases in thickness as HFs enter the growth phase to extend deeper into the dermal layer, and becomes thinner when HFs regress and enter the quiescent phase. While the hypodermis has been reported to secrete factors that control the HF growth cycle, it remained unknown whether, conversely, the HFs regulate adipogenesis or thickness of the hypodermis.
Now, a new study by Hironobu Fujiwara (Team Leader, Laboratory for Tissue Microenvironment) and his former colleagues at the Cancer Research UK Cambridge Research Institute, in collaboration with scientists at Kings College London, reveals that activation of the canonical Wnt/β-catenin signaling in the epidermis, which is known to promote HF growth, induces adipocyte differentiation in the hypodermis. Published in the Proceedings of the National Academy of Sciences, they show that epidermal Wnt/β-catenin signal regulates morphogenesis of the hypodermis by triggering the secretion of adipogenic factors, synchronizing the HF growth cycle with adipocyte differentiation.
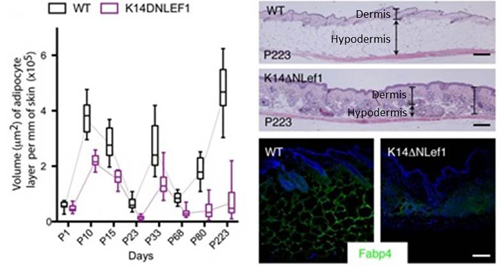
Because activation of the epidermal Wnt/β-catenin signaling pathway is known to induce HFs to enter the growth phase, the research group turned their attention to this signal to determine its effects on the hypodermis. They first generated a transgenic mouse, in which the epidermal Wnt/β-catenin signaling was blocked. During embryonic skin development, HF growth appeared normal in this mouse, but the hypodermis was thinner overall than that of wildtype. There was, however, an increase in the thickness of the reticular dermis, the lower layer of the dermis, which appeared to compensate for the thinner hypodermis. In the adult mutant mouse, adipocyte progenitor numbers were normal, but there was a significant decrease in the thickness of the differentiated adipocyte layer. Therefore, epidermal Wnt/β-catenin signaling is important for hypodermal adipocyte differentiation, and when blocked, limits the differentiation of adipocyte progenitors to mature adipocytes.
The group next analyzed the effects of activating the epidermal Wnt/β-catenin signal in the hair growth cycle, and found that HFs in the rest phase were induced to enter the growth phase and the hypodermis showed simultaneous expansion when Wnt/β-catenin was activated. While there were no changes in the number of adipocyte progenitors, they did see a rise in the thickness of the differentiated adipocyte layer. And when epidermal Wnt/β-catenin signal was activated in a transgenic mouse with impaired HF development, they observed that adipogenesis could be induced even in the absence of HFs, indicating that Wnt/β-catenin signal acts directly on the dermal layer to induce adipogenesis, not indirectly via HF formation.
Because the epidermis and dermis are separated by a basement membrane, it was thought that the epidermal Wnt/β-catenin signal is conveyed to the subdermal layer via a factor secreted by the epidermis. After reanalyzing gene expression profile data of previously reported transgenic mice with induced activation of epidermal Wnt/β-catenin signal, the group discovered that genes related to extracellular regions were upregulated in the epidermis, and genes related to fat metabolism, differentiation, and proliferation were upregulated in the underlying dermis. Thus, the analyses suggest that a secreted factor is produced from the epidermis, and that responses such as adipocyte proliferation and differentiation are seen in the dermis when epidermal Wnt/β-catenin is activated.
To confirm whether the epidermis secretes adipogenic factor(s), the group cultured adipocyte progenitors in medium conditioned with epidermis from different transgenic mice. In medium conditioned with epidermis in which Wnt/β-catenin activation was induced, more progenitors entered adipogenesis prematurely than seen under WT conditions, and in medium conditioned with Wnt/β-catenin–inhibited epidermis, very few adipocytes were generated. These results indicated that epidermal Wnt/β-catenin signal triggers the secretion of an adipogenic factor from the epidermis, which in turn induces adipocyte differentiation. They tested possible candidate factors gleaned from the gene expression profile data, and identified three factors (BMP2, BMP6, and Igf2) secreted by the epidermis that triggered adipocyte differentiation.
“Our study demonstrates that epidermal Wnt signal regulates adipogenesis in the hypodermis, and synchronizes adipocyte differentiation with HF growth cycle,” says Fujiwara. “For growing HFs to extend below the skin, additional space needs to be created in the dermal layer to accommodate the downgrowth. It is possible that inducing adipocyte differentiation, which has high volume expansion rates, is an efficient way for HFs to accomplish this.”
| Link to article | Epidermal Wnt/β-catenin signaling regulates adipocyte differentiation via secretion of adipogenic factors |
|---|