
News and Announcements from the CDB
Sound is generated by vibrations of air molecules, which are detected and interpreted by the ear. These vibrations are transduced, in turn, from the ear’s tympanic membrane, to the ossicular bones in the inner ear, to the perilymph in the spiral-shaped cochlea, and finally to the mechanosensory stereocilia of the hair cells (HCs). HCs then send electrical signals to the brain to be interpreted into what we perceive as sound. HCs in the cochlea are highly organized, with the mammalian cochlea comprising three rows of outer hair cells (OHCs), a single row of inner hair cells (IHCs), and populations of support cells that surround the HCs. Damage to the HCs by extrinsic or intrinsic factors can lead to hearing impairments, and once damaged, the hair cells cannot regenerate themselves. The mechanisms of HC development have long been studied as deciphering this process may lead to inroads in generating new HCs to replace damaged ones.
In a recent study, Kazuya Ono and colleagues in the Laboratory for Sensory Development (Raj K. Ladher, Laboratory head) shed new light on the role of one signaling pathway, the fibroblast growth factor (FGF) pathway, in cochlear HC development. Published in PLOS Genetics, they demonstrate that this pathway plays a crucial role in the maintenance of pre-hair cell progenitors and in the commitment of progenitors to differentiate into hair cells.
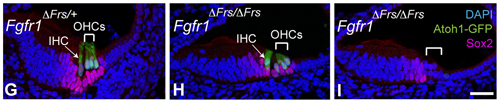
HCs form from an area of the inner ear epithelia which is induced to express Sox2, a transcription factor involved in maintaining pluripotency, by Notch-Jagged (Jag) 1 signaling. BMP signaling then specifies a region of cells within the Sox2-positive patch, also called sensory patch, to form the prosensory domain. These are the immediate precursors of HCs and support cells. The prosensory domain cells subsequently stop proliferating and a second wave of Notch signaling selectively induces the prosensory domain to differentiate into HCs or support cells. In addition to these pathways, studies by others have shown that the FGFR1 pathway is also important in cochlear HC development, as deleting the Fgfr1 gene resulted in HC loss. However, the question remained: how does this signaling pathway function in HC development.
Ono and his colleagues decided to focus on addressing this question by first determining when this signal is needed by reducing Fgfr1 levels at different stages in mice and noting its effects. They used two Fgfr1 conditional mutant mouse lines, which differed in the timing of Fgfr1 deletion; in the first line, Fgfr1 deletion occurred just prior to E10.5, before the induction of the sensory patch, and in the second line, the gene was deleted at E12.5. HC loss was observed in both lines; however, the loss was more dramatic when Fgfr1 levels were reduced at E10.5, suggesting that FGFR1 signaling was needed before E12.5.
Having established that FGFR1 signaling is needed before E12.5, the group next turned their attention on determining which step was disrupted. The defect was traced back to the earliest step in HC development, the formation of the Sox2 positive sensory patch. They found that while Sox2 was switched on normally, in the absence of FGF signaling, Sox2 was not maintained. Thus, the FGFR1 signaling pathway appears to be important for maintaining Sox2 expression during sensory patch formation, but is not involved in initiating the exiting of the cell cycle. Further analysis of the FGFR1 signaling pathway also revealed that the MAP kinase signaling cascade, acting through Frs2/3, is turned on downstream of FGFR1 and regulates Sox2 expression.
“In this study, we answer the longstanding question of how FGF signals regulate the hair cell numbers,” says Ladher. “It may be possible in the future to produce hair cells in culture, which can then be transplanted to replace the damaged inner ear hair cells, by modulating FGF signals.”
| Link to article | FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation |
|---|