
News and Announcements from the CDB
Formation of the complex neural network involves neurons extending a single axon to target and synapse with a dendrite sprouting from another neuron, which will in turn synapse with the next neuron. Some axons can extend up to one hundred times the length of the cell body to reach their target. The axons of neurons making up the amygdala, which is located deep in the middle of the brain mass, migrate along the hippocampus, making a long journey to reach the hypothalamus. These extending axons move collectively by forming fascicles (or bundles) consisting of several hundred to several thousand axons derived from the same neuronal group, but the underlying mechanism regulating fascicle formation and collective axonal migration remained unclear.
In a new study published in Developmental Cell, research scientist Shuichi Hayashi of the Laboratory for Cell Adhesion and Tissue Patterning (Takeichi Masatoshi, Team Leader) and colleagues identify one factor that plays an essential role in the collective axon extension of amygdala neurons. Using the developing mouse brain as a model, their findings indicate that protocadherin-17 (Pcdh17), a member of the cadherin superfamily of cell adhesion molecules, mediates axon-axon interactions and recruits actin regulators to the interaxonal contact sites which modify the surrounding actin dynamics to promote cell migration.
Protocadherins are broadly classified as being either clustered or non-clustered. Past studies suggest that clustered protocadherins have some function in the self-repelling mechanism of dendrites to prevent binding between dendrites from the same neuron, while non-clustered protocadherins have been implicated as a crucial player in axon elongation during neural network formation in the brain. In this study, Hayashi et al. focused their attention on a non-clustered protocadherin, Pcdh17, and its expression in amygdala neurons. Pcdh17 expression was detected in several subsets of amygdala neurons. Upon close examination, they found that Pcdh17 expression in these neuronal subsets was localized around the interaxonal adhesion sites, leading them to consider the role of Pcdh17 in the collective elongation of axons in amygdala neurons.
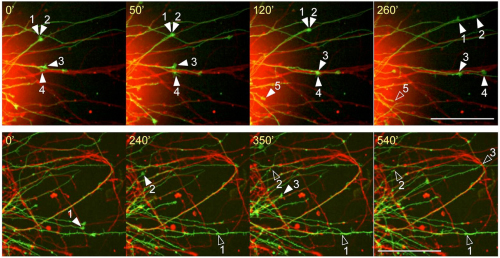
They next generated a Pcdh17 knockout mouse line, and an analysis of its phenotype revealed that loss of Pcdh17 results in abnormal axonal elongation; the axon fascicle extending from the amygdala to the hypothalamus was thinner. The thinner fascicle was due to a collapse of the growth cones, the motor center located at the tip of growing axons. Labeling the Pcdh17 expressing and non-Pcdh17 expressing neurons with different colored fluorescent proteins, the group observed growth cone migration using live-imaging and found that, in wild-type conditions, when the elongating axons came into contact with an axon from other neurons of the same subtype, it continued to elongate along the other axon, whereas in the Pcdh17 mutant, the axon stopped elongating when it came into contact with another neuron of the same subtype.
So then, how is the migration of axons regulated? Hayashi et al. confirmed that, similar to other cadherins which mediate intercellular interactions, the extracellular domain of Pcdh17 facilitates the binding of axons of similar neuronal types. Using a pull-down assay, they next looked into which molecules bind to the intracellular domain of Pcdh17 and identified several factors that make up the WAVE complex, which is involved in actin polymerization. They also determined that Pcdh17 works together with the WAVE complex to recruit other downstream factors that plays a role in cell motility of the growth cone as well as at the interaxonal adhesion sites. The role of Pcdh17 mediating cell migration was further verified by observing the behavior of Pcdh17-transfected cultured cells that showed similar directional movements to neuronal growth cones. Thus, the results showed that Pcdh17 promotes cell migration by localizing at interaxonal adhesion sites and recruiting the WAVE complex and further downstream factors, which enhance the efficiency of axon elongation.
“The functions of cadherins largely differ depending on the molecules it coordinates with,” explains Takeichi. “Neuronal axons can extend on their own, but when they form a fascicle, the contact enhances axon migration. Recent research shows that malfunction of protocadherins can lead to autism and schizophrenia. As the amygdala is a part of the brain that functions in emotion and memory, future studies looking at the relationship between protocadherins and amygdala will be very interesting.”
| Link to article |
|---|