
News and Announcements from the CDB
Oocytes display a surprisingly high frequency of errors in cell division despite being a vital means for animals, including humans, to pass on their genes to the next generation. During meiotic cell division in oocytes, sister kinetochores (KTs), one located on each chromosome making up a homologous chromosome pair (bivalents), must correctly form stable attachments to the microtubules (MT) and spindle apparatus for the bivalents to be properly segregated into two future daughter cells. Errors in the chromosomal segregation process in oocytes are known to cause congenital diseases such as Down syndrome or lead to miscarriages in humans. The mechanisms and the reasons for the high rate of errors in chromosome segregation in oocytes remain largely unknown.
Now a new study by research scientist Shuhei Yoshida and colleagues in the Laboratory for Chromosome Segregation (Tomoya Kitajima, Team Leader) has uncovered one of the causes of the high rate of errors seen in the first meiotic division (MI) in oocytes. They carried out a detailed analysis of the events during MI in mouse oocytes, focusing specifically around the KT-MT attachment sites, and discovered that correct KT-MT attachments are initially relatively unstable due to the prolonged presence of Aurora B and C (B/C) kinase, an attachment destabilizer, around the KT-MT attachment sites, contrary to what is observed in mitosis. Their findings, published in Developmental Cell, suggest that the modified features of KTs and chromosomes in MI makes it difficult for the oocyte to pry the KTs spatially away from the influence of Aurora B/C, thus contributing to errors in chromosome segregation.
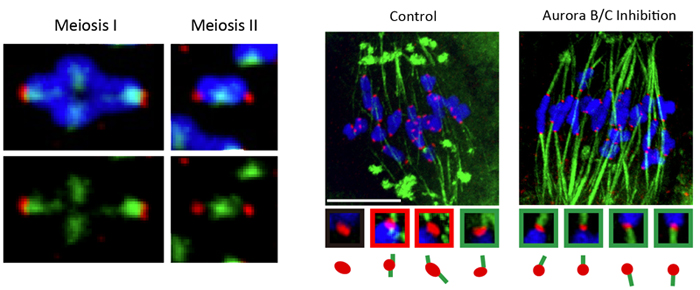
Left: Aurora B/C remains localized close to KT-MT attachment sites in MI than compared with MII, after chromosomes begin being pulled in opposite poles.
(red, kinetochores; green, microtubules; blue, chromosomes)
Right: Inhibiting Aurora B/C activity results in an increase in correct KT-MT attachments. (red, kinetochores; green, microtubules; blue, chromosomes).
In somatic cell division (mitosis), DNA is replicated to produce a pair of sister chromatids, which are then evenly divided into two daughter cells when the cell divides, whereas the first meiotic division involves the separation of pairs of homologous chromosomes (bivalents) into two cells that each contain half the number of chromosomes of the original cell. Past studies indicate that, in mitosis, the MTs extending from opposite poles form attachments to a pair of sister KTs in the inner centromere of the chromosomes and then physically pull apart the KTs, causing the phosphorylating Aurora B/C which localizes to the inner centromere to be spatially separated from the KT; this leads to a drop in phosphorylation levels of KTs, which stabilizes the KT-MT attachments. In meiosis, a stabilizing mechanism similar to that of mitosis is thought to be at work despite the inherent differences in the type of KT-MT attachments formed (bipolar versus monopolar), but there are no reports of studies that examined the MI events to confirm this belief. Yoshida et al. decided to focus on the stabilizing mechanism for KT-MT attachments in oocytes during MI.
Both mitosis and meiosis require the formation of correct and stable attachments of KTs to MTs to ensure the proper segregation of chromosomes. Past studies of mitosis suggest that stability of KT-MT attachments are linked to the regulation of KT phosphorylation, such as by Aurora B/C kinase which is known to localize to the inner centromere. In mitosis, MTs extending from opposite spindle poles form an attachment to KTs on the sister chromatids (bipolar attachments); the resulting tension draws the sister KTs apart, causing Aurora B/C kinase to become spatially separated from the KTs, and hence, stabilizing the attachment site. For MI of meiosis, MTs extending from the same pole form attachments with KTs on the bivalents (monopolar attachments), but the mechanisms at work to ensure correct, stable KT-MT attachments has not been closely examined. Despite the inherent differences in KT-MT attachments, a stabilizing mechanism similar to that seen in mitosis has been proposed for KT-MT attachments in MI.
Yoshida et al. first turned their attention to the localization of KT-MT attachment destabilizer Aurora B/C kinase during meiosis. During early MI, MTs emanating from an apolar spindle attach to sister KTs on the bivalents and the spindle then begins to bipolarize causing the bivalents to be pulled and stretched in opposite directions, eventually forming a belt-like spatial arrangement. But, at this point in time, the KT-MT attachment remains unstable. The team found that the Aurora B/C remained in the vicinity of the KT-MT attachment region even after the KTs were pulled apart by the MTs. In contrast, during meiosis II (MII), which unfolds in a manner similar to mitosis, Aurora B/C kinase was localized at some distance from the KT-MT attachment sites when the KTs were being pulled apart. When the oocytes were treated with an Aurora B/C kinase inhibitor as bivalents are stretched in MI, there was a marked rise in the number of correct KT-MT attachments formed, suggesting that the lingering Aurora B/C around the KT-MT attachment sites after bivalent stretching, as seen in MI, is a direct cause of the instability of KT-MT attachments.
Then what is the mechanism in MI that leads to the stability of the KT-MT attachments in later stages of MI? The group examined the changes in phosphorylation levels of the KT-MT attachments during MI and noticed that high phosphorylation levels were detected for a length of time after bivalent stretching, which then dropped markedly four to six hours later. These results suggested the workings of a molecular mechanism in MI that decreases phosphorylation levels at KT-MT attachment sites over a prolonged period leading to a gradual stabilization of these attachments without having to physically distance Aurora B/C from the KT-MT attachment sites.
To search for the factor that blocks the phosphorylating activity of Aurora B/C around KT-MT attachments, Yoshida et al. closed in on PP2A-B56, a phosphatase known to antagonize Aurora B/C activity by dephosphorylating KTs during mitosis. When localization patterns of PP2A-B56 during MI were analyzed, they found that its concentration levels gradually increased over several hours around the KT-MT attachment sites. They next searched for the mechanism underlying the regulation of PP2A-B56 levels and found that Cdk1-dependent phosphorylation of BubR1, known in mitosis as a checkpoint protein, assists in the recruitment of the phosphatase to the vicinity of KTs. The gradual accumulation of PP2A-B56 at KTs counters Aurora B/C activity, resulting in dephosphorylation of KT and progressive stabilization the KT-MT attachment sites.
“Our study has revealed the surprising instability of chromosome segregation in oocytes, and also a mechanism for stabilizing KT-MT attachments that is different from mitosis,” says Kitajima. “As the oocyte is the starting point of development, it has evolved many unique features. But we may be looking at an example of a biological paradox, if these features have unwittingly created a weak spot in basic intracellular activity.”
| Link to article |
Inherent Instability of Correct Kinetochore-Microtubule Attachments during Meiosis I in Oocytes |
|---|