
News and Announcements from the CDB
Almost all cell types display some sort of polarity, resulting in a diversity of cell shapes and functional differentiation. Extensive and robust changes in morphology can arise from polarization of cellular components during development, which requires tightly regulated intracellular transport systems capable of shuttling and localizing proteins and other molecular cargo to specific destinations within the cell. One widely observed cell morphogenetic phenomenon is cell elongation, in which a cell elongates in one direction during its development. The distal tip of an elongating cell serves as a hub for the signaling complexes that regulate molecular cargo trafficking as well as cytoskeletal organization. While motor proteins are known to shuttle molecular cargo to the distal tip along the polarized cytoskeleton, how the signaling molecules that coordinate elongation are localized and maintained at the tip during elongation remain poorly understood.
Now, a new study published in Development by Tetsuhisa Otani in the Laboratory for Morphogenetic Signaling (Shigeo Hayashi, Team Leader) and his colleagues offers new insights into how distal tips of elongating cells are organized and maintained. Using the mechanosensory bristle of the Drosophila fruit fly as a model system, they demonstrate a mechanism regulating the correct transport and positioning of IκB kinase ε (IKKε), a protein kinase that plays a central role sorting molecular signals needed for elongation, near the distal tip of the elongating bristle.
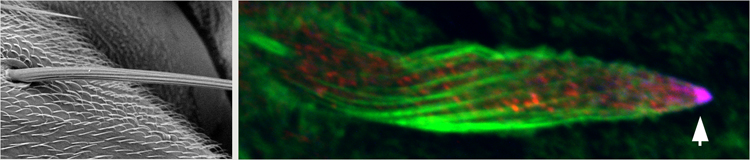
Left: Electron microscopy image of Drosophila bristle. Right: Pupal stage bristle cell. IKKε (blue) and Spn-F (red) are colocalized near elongating tip (white arrow).
In a previous work also led by Otani using the same model, they reported that once IKKε was localized and activated at the distal tip, it acts as a signaling center to sort the various molecular cargos transported to the tip (see CDB News: February 16, 2011). To solve the mystery of how IKKε is robustly maintained at the distal tip during elongation, the group combed through past publications for molecules that reportedly bind to IKKε.
Their search led them to the Spindle-F (Spn-F) protein, which has been shown to interact with IKKε as well as contribute to the localization of active IKKε. These two proteins were speculated to function as a pair due to similar polarity defects observed in oocytes of spn-F and of ikkε mutants. Upon closer examination of the respective mutants, Otani et al. discovered bristle morphology defects in both mutants; bristles were shorter, with some displaying multiple branching near the tip or bulges along the bristle. In normal bristles, activated IKKε and Spn-F are found co-localized at the tip of the bristle, but Spn-F is also found dispersed in the cytoplasm. Time-lapse imaging experiments tracking cytoplasmic Spn-F behavior revealed that the protein moved along microtubules to the tip, and fluorescence recovery after photobleaching (FRAP) experiments also showed that Spn-F was stably localized to the distal tip once transported there.
How then are IKKε and Spn-F transported to the tip? Cytoplasmic dynein, a minus-end motor protein, was a prime candidate because microtubules in the elongating tip are oriented with the minus-ends facing the distal tip. Indeed, when dynein activity was inhibited in the bristle, both IKKε and Spn-F were unable to localize to the distal tip. When the domain regions of the Spn-F were analyzed, they found that cytoplasmic dynein and IKKε could bind simultaneously to Spn-F through distinct binding regions and that this simultaneous binding is crucial for cell elongation. Thus, Spn-F appears to serve as an adaptor protein linking IKKε to dynein, which transports them both along the microtubules to the distal tip.
Next, Otani et al. turned their attention to the mechanism underlying the sustained localization of IKKε at the tip after being transported there by cytoplasmic dynein. They examined the possible role of a protein called Javelin-like (Jvl) at the distal tip as it is known interact with Spn-F and also contributes to the polarization of active IKKε in oocytes. In jvl mutants, IKKε and Spn-F colocalized at the distal tip during early stages of elongation, but in the later stages, they were no longer localized at the tip, and bristle tips showed morphological defects. Time-lapse imaging tracking the molecular dynamics of Jvl and Spn-F revealed that each molecule actively moves along the microtubules independently of the other, but when both proteins colocalize together, they stop moving. The interaction of Jvl and Spn-F thus results in their immobilization, and consequently immobilizes IKKε, which is bound to Spn-F.
Based on their findings, they proposed that the IKKε-Spn-F complex and Jvl are transported independently to the distal tip in a unidirectional manner, and when Jvl interacts with Spn-F at the tip, the IKKe-Spn-F complex becomes anchored there.
“Spn-F is not just an adaptor molecule linking the motor protein to IKKε. It also serves as a signal to recruit factors that regulate dynamics of IKKε after being transported to the tip,” says Hayashi. “Examining different adaptor molecules may help us understand the elaborate intracellular transport system underlying the morphogenesis of more complex cell shapes such as mammalian neurons.”
| Link to article | |
|---|---|
| Related Link |