
News and Announcements from the CDB
Periodic structural patterns, as seen in centipedes or woodlice, are fundamental body pattern forms that are also commonly observed in humans and other animals. How these periodic structural patterns arise is one enduring fundamental question of developmental phenomena. In the 1950s, Alan Turing, a brilliant British mathematician and founder of the concept for modern computing, proposed that a reaction between two molecules diffusing at different speeds could produce a striped pattern autonomously without preexisting positional information, and that this molecular level self-organization was the basis of morphogenesis in living organisms. Many groups have since attempted to validate his theory through biological experiments, and to date it has been demonstrated at the cellular level to produce striped patterns in fish. However, little progress has been made to validate the theory in vivo at the molecular level.
New work by former research scientist Bo Dong in the Laboratory for Morphogenetic Signaling (Shigeo Hayashi, Team Leader), now a professor at the Ocean University of China, in collaboration with Edouard Hannezo at the Institut Curie in France reveals a self-organizing mechanism of actomyosin in the cell cortex that produces the actin ring patterns in the tracheal tube of Drosophila. They demonstrate through mathematical modeling and experimental observations that actin ring patterns are self-organized by actin flows created by myosin contractility-induced forces and that these actin rings are stabilized by extracellular mechanical factors. Their study, published in Proceedings of National Academy of Sciences (PNAS), establishes the relevance of Turing’s theory in understanding biological phenomena.
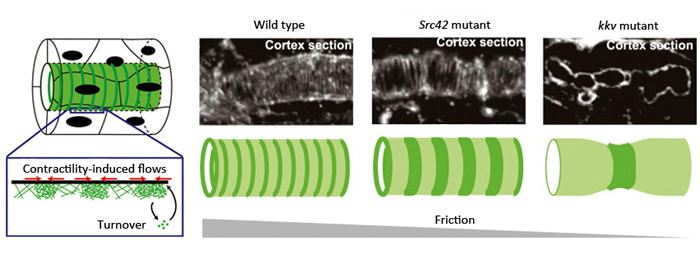
Left: Model of actin ring formation. Right: Actin rings formed along the cortex of tracheal tube. In mutants for a factor involved in stabilizing adhesion complex (Src42 mutant) and those unable to produce ECM (kkv mutant), cortical friction decreased and intervals between actin rings were wider than seen in wild type. This is consistent with predictions of the mathematical model.
In Drosophila tracheal tube formation, the epithelial cells are aligned with the apical side facing interior or lumen of the tube. As the lumen begins to form, evenly spaced actin rings are generated along the tracheal tube, straddling the apical cell cortex (just below the cell membrane) of epithelial cells. A hard cuticular structure is then assembled around the actin rings of the tracheal tubes after the larva hatches from the egg. Similar periodic structures are also seen in mammalian trachea and C. elegans embryos, which suggests that this is a fundamental mechanism for maintaining tubular or cylindrical structures.
How then is the actin ring pattern formed? To maintain the evenly spaced patterns beyond cell boundaries, the team speculated that there must be a mechanism functioning at the tissue level. Hannezo and Recho adopted a theoretical approach using mathematical modeling to predict that in a viscous cytoplasmic environment, the repeated contractility-driven forces of the myosin motor protein causes actin flows, eventually giving rise to a periodic actin ring pattern.
Dong then set out to validate the model experimentally. They first analyzed the progression of Drosophila tracheal tube development using fluorescence live imaging, which revealed a gradual increase in actin concentration and appearance of circumferential supracellular actin rings, each cell with 15 to 20 of these rings. Because myosin II is known to colocalize with actin, the rings were speculated to arise from the binding of actin and myosin II to form the contractile actomyosin complex. They next used fluorescence recovery after photobleaching (FRAP) experiments to confirm that actin flow and turnover did occur in the cortex. When the developing embryo was treated by a drug inhibiting myosin contractility, the actin rings disappeared, indicating the requirement of myosin activity for actin ring formation in the trachea. Thus, together these experiments validated that the basic assumptions of the mathematical model were supported in vivo.
In their proposed model, friction within the cell cortex was postulated as a hypothetical factor influencing actin flows, playing a large role in orienting or positioning actin within the cortex. It predicted that actin would be assembled in a circumferential ring pattern when circumferential friction was slightly higher than axial friction, and that a decrease in friction would lead to wider spacing between the actin rings. Thus, Dong et al. hypothesized that the source of friction was the binding between actin filaments, the intercellular adhesion complex and the cell cortex. They examined flies with mutations in a gene encoding a factor important for stabilizing the adhesion complex, and found that the intervals between the actin rings were wider than seen in the wild type. In another experiment where actin-cell cortex binding was inhibited, they also found wider spaced pattern of actin rings. These results were consistent with the predictions made by their model, and demonstrate that cell cortical friction contributes to ring pattern formation in the tracheal tube.
Another source of friction was considered to be the binding between the cell cortex and the solid extracellular matrix (ECM) that initially fills the tracheal tube. Chitin fibers, the main components of the ECM, are aligned in the tubule in an anteroposterior direction, distributed in a manner enabling directional friction. Dong et al. examined a fly mutant strain that cannot synthesize chitin and consequently has no chitin fibers in the developing tube, and observed that the contractile forces exerted by the actin ring was enough to cause localized constrictions of the tube. The spacing between the actin rings was uneven and irregular, causing the tube to kink in several locations and display non-uniform circumference along its length. These results suggest that under normal conditions where the ECM fills the lumen, friction between the ECM and the cell cortex contributes to the stabilization of actin rings and uniformity of the tracheal tube. Thus, the actin dynamics observed in the trachea tube are consistent with the behavioral predictions of their model when values for factors such as friction are manipulated.
“It was surprising to discover that molecular level self-organization, as proposed by Turing over 50 years ago, is one mechanism maintaining the uniform circumference of the tracheal tube throughout its entire length,” says Hayashi. “We hope to further investigate the physical laws associated with tissue level morphogenesis when supracellular structures that span the cells, such as actin rings, are controlled by extracellular factors.”
| Link to article |
Cortical instability drives periodic supracellular actin pattern formation in epithelial tubes |
|---|