
News and Announcements from the CDB
Embryonic stem cells (ESCs) are derived from cells extracted from the inner cell mass of early stage embryos. When cultured under the right conditions, they are capable of dividing and proliferating indefinitely, all the while retaining the potential to become any somatic cell in the body. In most somatic cells, the terminal ends of DNA called telomeres become shorter after each successive round of cell division. Once telomere shortening reaches a critical limit, the cell stops dividing, eventually progresses into senescence (aging) and finally cell death. The exceptions to this are germ cells, stem cells and cancer cells, which can repair their telomeres through telomere elongation and continue to proliferate. In ESCs, a DNA-binding protein called ZSCAN4 is known to play an important role in regulating telomere elongation and genomic stability; however, its gene is not expressed in all ESCs, and it was unclear what triggered its expression.
In a new study by research scientist Yoko Futatsugi and Team Leader Hitoshi Niwa in the Laboratory for Pluripotent Stem Cell Studies, they investigated the conditions leading to the activation of Zscan4 expression in mouse ESCs by monitoring its activity at the single cell level and found that it is activated in response to telomere shortening. They also discovered that the cell cycle lengths were longer when Zscan4 was expressed, presumably reflecting the time necessary for telomere maintenance. Their findings were published in the journal Stem Cell Reports.
Futatsugi and Niwa first transfected mouse ESCs with two transgenes, each expressing different colored fluorescence when activated. They then established an experimental system where a nuclear marker was used to identify and track individual cells as well as analyze the Zscan4 activity quantitatively over time. Individual mouse ESCs were tracked over a period of 120 hours, and a cell lineage tree was constructed based on the tracking data. The team also analyzed the cell-cycle length, and telomere length in these cells, and looked for correlations between these variables and Zscan4 expression levels.
They were surprised to find that the cell-cycle length of mouse ESCs, which were thought to be approximately 12 hours, in fact displayed considerable variation, ranging between 10–30 hours. When they examined whether a correlation existed between cell-cycle length and Zscan4 expression levels, cells with longer cell cycles were discovered to show higher expression levels of Zscan4. Those cells with high Zscan4 expression levels also had shorter cell-cycle lengths in the following round of cell division. Searching for a link between cell-cycle length and telomere length, they found that cells with longer cell cycles had shorter telomeres. Thus, these results strongly suggested that as the ESCs undergo successive rounds of cell division, their telomeres become shorter, which triggers activation of Zscan4 expression. Zscan4 activation, in turn, functions to elongate the shortened telomeres, resulting in extended cell-cycle lengths.
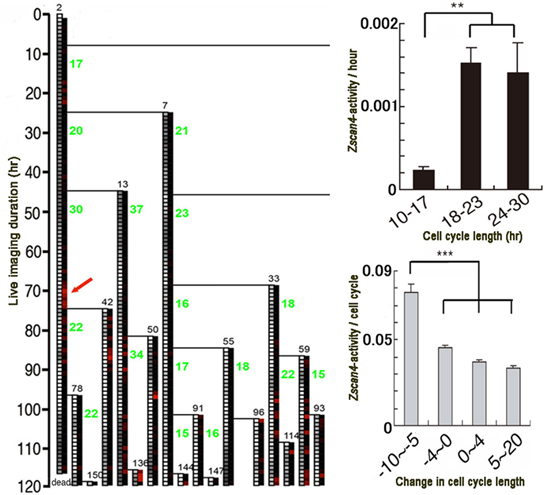
Left: Example of cell lineage tree. Cells were sequentially numbered in the order they emerged (small black numbers).
Numbers in green indicate cell cycle length (hours). Zscan4 activity is indicated on a scale of red intensity.
Cells expressed Zscan4 transiently. Top, right: Zscan4 activity is higher when cell cycle length is longer. Bottom, right:
When Zscan4 expression levels are high, the next round of cell division tends to be shorter.
Futatsugi and Niwa also examined the relationship between pluripotency marker gene Rex1 and expression of Zscan4, but they did not find any correlations between these two factors. This suggested that telomere elongation by Zscan4 functions independent to the mechanism maintaining ESC pluripotency.
“ESCs are known to be a heterogeneous population of cells. In this study, our single-cell tracking method uncovered surprising revelations about the telomere elongation mechanism that could not be identified simply through examinations of ESC colonies,” explains Futatsugi. “As Zscan4 appears to be activated through some mechanism sensing telomere length, our next step is to try understanding the details of this regulatory mechanism. We hope that further unveiling of innate characteristics of ESCs will lead to the development of more stable culturing methods of pluripotent stem cells, including iPS cells.”
| Link to article |
Zscan4 is activated after telomere shortening in mouse embryonic stem cells. |
|---|